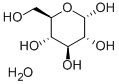
2-Deoxy-D-glucose
Synonym(s):2-Deoxy-D -arabinohexose;2-Deoxyglucose
- CAS NO.:154-17-6
- Empirical Formula: C6H12O5
- Molecular Weight: 164.16
- MDL number: MFCD00151328
- EINECS: 205-823-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16

What is 2-Deoxy-D-glucose?
Description
2-Deoxy-D-glucose (154-17-6) is a synthetic glucose analog with extensive biological effects. It is commonly thought of as an inhibitor of glycolysis, but its metabolic effects are wide-ranging. 2-Deoxy-D-glucose competitively inhibits glucose uptake via its metabolite 2-Deoxy-D-glucose-6-phosphate, which inhibits hexokinase and phosphoglucose-isomerase leading to decreased ATP production, cell cycle blockage, decreased cell growth and ultimately cell death.1,2
Chemical properties
white to light yellow crystal powde
The Uses of 2-Deoxy-D-glucose
2-Deoxy-D-glucose (2-DG) is a versatile glucose analog with significant applications in both research and therapeutic development. It is utilized in glucoprivic feeding studies to invoke and examine the counter-regulatory response (CRR), a critical metabolic adaptation to low glucose conditions. In the realm of molecular genetics, 2-DG serves as a component of culture media and as a targeted optical imaging agent for fluorescent in vivo imaging. Beyond its role in research, 2-DG is also integral to the development of anti-cancer strategies, particularly those involving oxidative stress, radiotherapy, and chemotherapy sensitization. Functioning as a metabolic inhibitor, 2-DG blocks the phosphorylation of glucose by hexokinase, the initial step in glycolysis, leading to a cascade of effects including cellular ATP depletion, inhibition of protein glycosylation, and ER quality control disruption through the induction of the unfolded protein response. These actions contribute to cell cycle inhibition, cell death under hypoxic conditions, autophagy induction, reactive oxygen species production, AMPK activation, and ultimately, the blocking of tumor cell growth in animal models, showcasing 2-DG's potential as a multifaceted tool in both scientific inquiry and medical intervention.
What are the applications of Application
2-Deoxy-D-glucose is an apoptosis and HXK inhibitor that lowers ATP levels in cancer cell lines and has shown to be taken up by glial cells incubated with insulin
Biochem/physiol Actions
2-Deoxy-D-Glucose (2-Deoxyglucose) is a glucose analog that inhibits glycolysis via its actions on hexokinase, the rate limiting step of glycolysis. It is phosphorylated by hexokinase to 2-DG-P which can not be further metabolized by phosphoglucose isomerase. This leads to the accumulation of 2-DG-P in the cell and the depletion in cellular ATP. In vitro, 2-Deoxyglucose has been shown to induce autophagy, increase ROS production, and activate AMPK.
Safety Profile
Poison by subcutaneous route. Moderately toxic by intraperitoneal route. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits acrid smoke and fumes.
Synthesis
Glucal is the glycal formed from glucose and is one of the common starting materials for the synthesis of 2-deoxy-d-glucose (2-DG). A general conversion involves the bromination (or halogenation) of Glycal at C-2 followed by the replacement of bromine with hydrogen. Bromination takes place in nucleophilic solvent using molecular bromine. Masuda and coworkers reported the synthesis of 2-DG from D-glucose. 2-Deoxy-D-glucose was prepared in three steps from natural D-glucose dispensing with any protection/deprotection procedure and was obtained in 48% yield.
Toxicity
Toxic effects of 2-deoxy-d-glucose (2-DG) result from its ability to block glycosylation but not glycolysis. Ketogenic Diet increases tolerance against glycolysis inhibitors. Experimental results show that 2-DG is a relatively harmless compound at low doses but can lower blood pressure and slow breathing at higher doses. Most studies indicate that a clinically tolerable dose of 2-DG is up to 63?mg/kg/day. Beyond this, several side effects are observed, including reversible hyperglycemia, gastrointestinal bleeding, and QTc prolongation. However, recent studies carried out by DRDO use a higher dose of 90?mg/kg/day. Exposure to 2-DG causes cytotoxicity and radiosensitization via a mechanism involving changes in thiol metabolism, and these effects may be more prominent in transformed vs. normal cells.
storage
Store at +4°C
References
1) Ralser et al., (2008), A catabolic blockade does not sufficiently explain how 2-deoxy-D-glucose inhibits cell growth; Proc. Natl. Acad. Sci. USA 105 17807 2) Giammarioli et al. (2012), Differential effects of the glycolysis inhibitor 2-deoxy-D-glucose on the activity of pro-apoptotic agents in metastatic melanoma cells; Int. J. Cancer, 131 e337
Properties of 2-Deoxy-D-glucose
| Melting point: | 146-147 °C(lit.) |
| Boiling point: | 211.61°C (rough estimate) |
| alpha | 45.5 º (c=2, H2O) |
| Density | 1.1738 (rough estimate) |
| refractive index | 46.5 ° (C=1, H2O) |
| storage temp. | 2-8°C |
| solubility | H2O: 50 mg/mL, clear, colorless to faintly yellow |
| form | crystalline |
| pka | pK1:12.52 (25°C) |
| color | white |
| Water Solubility | Soluble in water. |
| Merck | 14,2904 |
| BRN | 1723331 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. Solutions in water are not stable and must be used within 1 working day. |
| CAS DataBase Reference | 154-17-6(CAS DataBase Reference) |
| EPA Substance Registry System | D-arabino-Hexose, 2-deoxy- (154-17-6) |
Safety information for 2-Deoxy-D-glucose
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 2-Deoxy-D-glucose
| InChIKey | PMMURAAUARKVCB-CEZCPVKQSA-N |
2-Deoxy-D-glucose manufacturer
Synaptics Labs Private Limited
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 2-DEOXY-D-GLUCOSE 98%View Details
2-DEOXY-D-GLUCOSE 98%View Details -
 2-Deoxy-D-glucose CAS 154-17-6View Details
2-Deoxy-D-glucose CAS 154-17-6View Details
154-17-6 -
 2-Deoxy-D-Glucose (2-DG) extrapure AR CAS 154-17-6View Details
2-Deoxy-D-Glucose (2-DG) extrapure AR CAS 154-17-6View Details
154-17-6 -
 2-Deoxy-D-glucose CAS 154-17-6View Details
2-Deoxy-D-glucose CAS 154-17-6View Details
154-17-6 -
 2-Deoxy-D-glucose CAS 154-17-6View Details
2-Deoxy-D-glucose CAS 154-17-6View Details
154-17-6 -
 2-Deoxy-D-glucose CAS 154-17-6View Details
2-Deoxy-D-glucose CAS 154-17-6View Details
154-17-6 -
 2-Deoxy-D-glucose CAS 154-17-6View Details
2-Deoxy-D-glucose CAS 154-17-6View Details
154-17-6 -
 2-Deoxy-D-glucose CAS 154-17-6View Details
2-Deoxy-D-glucose CAS 154-17-6View Details
154-17-6