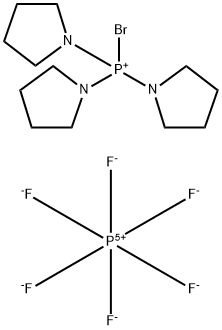
1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate
Synonym(s):Benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphoniumhexafluorophosphate, Castro′s Reagent;BOP;BOP Reagent;Castros reagent
- CAS NO.:56602-33-6
- Empirical Formula: C12H22F6N6OP2
- Molecular Weight: 442.29
- MDL number: MFCD00011948
- EINECS: 260-279-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
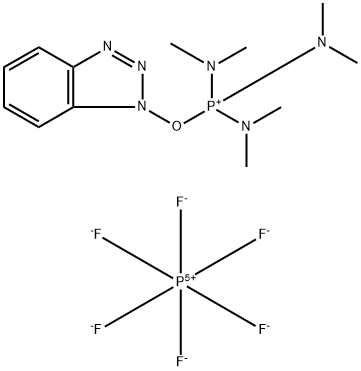
What is 1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate?
Description
BOP is a reagent used occasionally in amide coupling reactions. The use of BOP forms Hexamethylphosphoramide (HMPA) as a by-pdt which is a known carcinogen. PyBOP is considered a safer alternative to BOP.
Chemical properties
white to off-white powder
The Uses of 1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate
1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate is a peptide coupling reagent. Can be used in the preparation of phenyl esters of amino acids which have been shown to be valuable as blocked derivatives of amino acids in the field of peptide synthesis.
The Uses of 1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate

To a mixture of the acid (0.3 g, 0.85 mmol) and BOP (442 mg, 1 mmol) in DMF at 0 C was added TEA (0.3 mL 2.14 mmol). The resulting mixture was stirred for 5 min, after which time the amine (110 mg, 1 mmol) in DMF was added dropwise and the reaction was stirred at RT for 5 h. The mixture was diluted with H2O and stirred overnight. The resulting solids were filtered and purified by chromatography (3% MeOH/CHCl3) to provide the product. [70 mg]
The Uses of 1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate
Peptide coupling reagent which suppresses racemization.
The Uses of 1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate
1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate is used as a reagent for peptide coupling, lactonization, selective esterification, amidation of alfa amino acids without racemization and synthesis of magnolamide for antioxidative activity and catalyst for 9-acridinecaroboxamide derivative. It is also used as a precursor for the synthesis of phenyl esters of amino acids. It acts as a substitute for (Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate (BOP) reagent.
What are the applications of Application
BOP is a useful reagent for peptide coupling, esterification of carboxilic acids and other research uses
Preparation
To vigorously stirred HMPA (15.0 g, 83.7 mmol) at 0 C°, a solution of triphosgene (11.28 g, 38.01 mmol) in dichloromethane (15 mL) was added over a period of 40 min. The ice bath was then removed and the mixture was stirred at room temperature. At various intervals, small aliquots were removed in order to follow the disappearance of the HMPA spectroscopically. After 3 h, the solvent was removed under reduced pressure to leave a residue. This residue was redissolved in dry dichloromethane (40 mL) and solid hydroxybenzotriazole monohydrate (12.76 g, 94.4 mmol) was added with stirring. The resulting solution was cooled to about 5 C° with an acetone/ice bath, whereupon triethylamine (8.42 g, 83.4 mmol) was added over a period of 15 min and stirring was continued at -5 C for 4 h. The residue was dissolved in water (50 mL) and mixed with a filtered solution of potassium hexafluorophosphate (16.68 g, 90.6 mmol) in water (120 mL) to give benzotriazolyl-N-oxytris(dimethylamino)phosphonium hexafluorophosphate 1317 (BOP) as a crystalline solid (28.91 g, 78%).
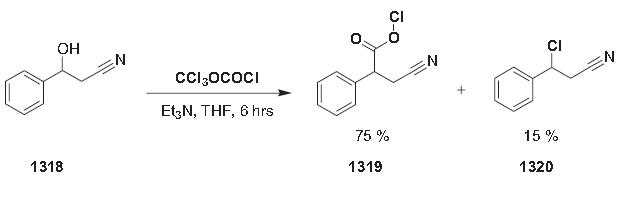
Chloroformates 1319 and chlorides 1320 are also formed when secondary benzyl alcohols 1318 are treated with trichloromethyl chloroformate (diphosgene) in the presence of triethylamine. The distribution of products can be controlled.
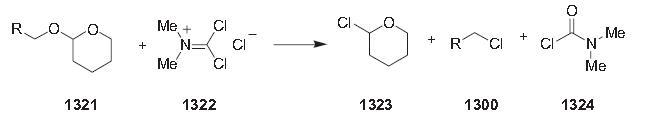
Reactions of tetrahydropyranylated alcohols 1321 with N,N-dimethylphosgeniminium chloride (‘‘Viehe salt’’) 1322 give the corresponding alkyl chloride 1300 in good yields [997]. This conversion can be conveniently accomplished by adding the ‘‘Viehe salt’’ (1.05 equiv.) as a solid to a solution of the THP-protected alcohol (1 equiv.) in anhydrous dichloromethane (0.3 m) under argon at 0 C°. After completion of the reaction and aqueous work-up, the crude alkyl chlorides are purified by column chromatography.
Purification Methods
Dissolve it in CH2Cl2, dry (MgSO4), filter, concentrate it under a vacuum, then add dry Et2O and filter off the first crop. Add CH2Cl2 to the filtrate and concentrate again to obtain a second crop. The solid is washed with dry Et2O and dried in a vacuum. Also recrystallise it from dry Me2CO/Et2O and check the purity by NMR. Store it in the dark. [Castro et al. Synthesis 751 1976, Nguyen et al J Chem Soc, Perkin Trans I 1915 1987, Coste et al. Tetrahedron Lett 36 4253 1995.]
Properties of 1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate
| Melting point: | >130 °C (dec.)(lit.) |
| Flash point: | 138°C |
| storage temp. | 2-8°C |
| solubility | methanol: 25 mg/mL, clear |
| form | Powder |
| appearance | White crystalline powder |
| color | White to off-white |
| Water Solubility | Soluble in methanol, acetone and dichloromethane. Insoluble in water. |
| Sensitive | Moisture & Light Sensitive |
| Decomposition | 138 ºC |
| BRN | 4287025 |
| CAS DataBase Reference | 56602-33-6(CAS DataBase Reference) |
Safety information for 1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H228:Flammable solids H315:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for 1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate
1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate manufacturer
JSK Chemicals
ASM Organics
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![5-Chloro-1-[bis(dimethylamino)methylene]-1H-benzotriazolium 3-oxide hexafluorophosphate](https://img.chemicalbook.in/CAS/GIF/330645-87-9.gif)

You may like
-
 56602-33-6 BOP reagent 98%View Details
56602-33-6 BOP reagent 98%View Details
56602-33-6 -
 56602-33-6 99%View Details
56602-33-6 99%View Details
56602-33-6 -
 56602-33-6 BOP Reagent, 95% 99%View Details
56602-33-6 BOP Reagent, 95% 99%View Details
56602-33-6 -
![1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate [Coupling Reagent for Peptide] CAS 56602-33-6](https://img.chemicalbook.in//Content/image/CP5.jpg) 1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate [Coupling Reagent for Peptide] CAS 56602-33-6View Details
1H-Benzotriazol-1-yloxytris(dimethylamino)phosphonium Hexafluorophosphate [Coupling Reagent for Peptide] CAS 56602-33-6View Details
56602-33-6 -
 (Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate, 97% CAS 56602-33-6View Details
(Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate, 97% CAS 56602-33-6View Details
56602-33-6 -
 BOP Reagent extrapure AR CAS 56602-33-6View Details
BOP Reagent extrapure AR CAS 56602-33-6View Details
56602-33-6 -
 BOP CAS 56602-33-6View Details
BOP CAS 56602-33-6View Details
56602-33-6 -
 (Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate CAS 56602-33-6View Details
(Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate CAS 56602-33-6View Details
56602-33-6
