Lithium hexafluorophosphate
Synonym(s):1.0 M LiPF6 DEC;1.0 M LiPF6 DMC;1.0 M LiPF6 EC/DEC=50/50 (v/v);1.0 M LiPF6 EC/DMC;1.0 M LiPF6 EC/EMC=50/50 (v/v)
- CAS NO.:21324-40-3
- Empirical Formula: F6LiP
- Molecular Weight: 151.91
- MDL number: MFCD00011096
- EINECS: 244-334-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-20 17:40:16
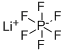
What is Lithium hexafluorophosphate?
Chemical properties
white crystalline powder
The Uses of Lithium hexafluorophosphate
Lithium hexafluorophosphate (LiPF6) is the most widely used solute in liquid and gelled-type electrolytes,which has good solubility in various solvents such as PC (propylene carbonate) .
The Uses of Lithium hexafluorophosphate
Used as an electrolyte in Li-ion batteries.Lithium hexafluorophosphate is used as an electrolyte in lithium batteries, ceramic industries and for welding electrode manufacturing. It is also used in commercial secondary batteries, prism spectrometer and x-ray monochromator. Further, it catalyzes the tetrahydropyranylation of tertiary alcohol.
The Uses of Lithium hexafluorophosphate
The solutions of LiPF6 in alkyl carbonates find applications as electrolytes in lithium ion batteries and the use of high quality battery grade electrolytes having extremely low water (<15 ppm) and hydrogen fluoride (<50 ppm) contents are critical for achieving high electrochemical performance.
Definition
ChEBI: Lithium hexafluorophosphate is an inorganic lithium salt having hexafluorophosphate(1-) as the counterion. It is an electrolyte used in lithium-ion batteries. It contains a hexafluorophosphate(1-).
General Description
Lithium hexafluorophosphate solution in ethylene carbonate and ethyl methyl carbonate is a class of electrolytic solution that can be used in the fabrication of lithium-ion batteries. Lithium-ion batteries consist of anode, cathode, and electrolyte with a charge-discharge cycle. These materials enable the formation of greener and sustainable batteries for electrical energy storage.
Flammability and Explosibility
Non flammable
Battery Materials
Lithium hexafluorophosphate (LiPF6) is the most widely used salt in the electrolytes for commercial Li-ion cells. It is commonly used as the electrolytic solution in lithium-ion rechargeable batteries. It is hydrolyzed by the small amounts of water contained in the electrolytic solution to produce fluoride and other ions.
According to the ionics studies on the limiting properties in various solvents, this excellent conductivity results from the combination of its ionic mobility and dissociation constant, although in neither category does LiPF6 stand at the most outstanding position:
Average ion mobility: LiBF4 > LiClO4 > LiPF6 > LiAsF6 > LiTf >
LiIm Dissociation constant: LiTf < LiBF4 < LiClO4 < LiPF6 < LiAsF6 < LiIm
The reversed order in the above two properties clearly demonstrates the conflicting nature of the requirements and the advantage of the well-balanced properties of LiPF6.
Lithium hexafluorophosphate is one of the most used electrolyte salt in the production of lithium ion batteries. Electrolyte Lithium Hexafluorophosphate for Lithium-ion Batteries has the ability of dissolving in binary and ternary solvents which cyclic carbonates and linear carbonates can be given as example. After lithium hexafluorophosphate dissolves in these solvents, it shows high electrolytic conductivity and thermal stability which is a desired property for lithium ion batteries.
Properties of Lithium hexafluorophosphate
| Melting point: | 200 °C (dec.) (lit.) |
| Density | 1.5 g/mL (lit.) |
| Flash point: | 25 °C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: slightly soluble(lit.) |
| form | Powder |
| Specific Gravity | 1.50 |
| color | APHA: <50 |
| Water Solubility | soluble |
| Sensitive | Hygroscopic |
| Exposure limits | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3 |
| Stability: | Stable, but readily hydrolyzes upon exposure to water or moist air. Incompatible with strong oxidizing agents, strong acids. |
| CAS DataBase Reference | 21324-40-3(CAS DataBase Reference) |
| EPA Substance Registry System | Phosphate(1-), hexafluoro-, lithium (21324-40-3) |
Safety information for Lithium hexafluorophosphate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H314:Skin corrosion/irritation H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lithium hexafluorophosphate
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Lithium hexafluorophosphate solution CASView Details
Lithium hexafluorophosphate solution CASView Details -
 Lithium hexafluorophosphate solution CASView Details
Lithium hexafluorophosphate solution CASView Details -
 Lithium hexafluorophosphate solution CASView Details
Lithium hexafluorophosphate solution CASView Details -
 Lithium hexafluorophosphate solution CASView Details
Lithium hexafluorophosphate solution CASView Details -
 Lithium hexafluorophosphate solution CASView Details
Lithium hexafluorophosphate solution CASView Details -
 Lithium hexafluorophosphate solution CASView Details
Lithium hexafluorophosphate solution CASView Details -
 Lithium hexafluorophosphate solution CASView Details
Lithium hexafluorophosphate solution CASView Details -
 Lithium hexafluorophosphate solution CASView Details
Lithium hexafluorophosphate solution CASView Details
