18-Crown-6
Synonym(s):1,4,7,10,13,16-Hexaoxacyclooctadecane;1,4,7,10,13,16-Hexaoxacyclooctadecane, 18-Crown-6;18C6;Crown ether/18-Crown-6
- CAS NO.:17455-13-9
- Empirical Formula: C12H24O6
- Molecular Weight: 264.32
- MDL number: MFCD00005113
- EINECS: 241-473-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:47
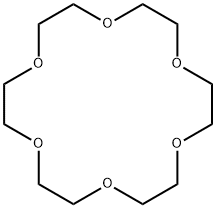
What is 18-Crown-6 ?
Chemical properties
slightly yellow solid
The Uses of 18-Crown-6
18-Crown-6 is used as an efficient phase transfer catalyst and as a complexing agent with a variety of small cation. It is involved in the synthesis of diaryl ethers, diaryl thioethers, and diarylamines mediated by potassium fluoride-alumina and 18-crown-6. It facilitates the solubility of potassium permanganate in benzene, which is used for oxidizing the organic compounds. It is used to accelerate various substitution reactions as well as enhances the power of nucleophiles such as potassium acetate. It is utilized in the alkylation reactions in the presence of potassium carbonate, N-alkylation of glutarimide and succinimide with dimethylcarbonate. The complex formed by its reaction with potassium cyanide acts as a catalyst in the cyanosilylation of aldehydes, ketones and quinines with trimethylsilyl cyanide (TMSCN). It may be used to catalyze the N-alkylation of heterocyclic compounds and allylation of functionalized aldehydes.
Definition
ChEBI: 18-crown-6 is a crown ether that is cyclooctadecane in which the carbon atoms at positions 1, 4, 7, 10, 13 and 16 have been replaced by oxygen atoms. It has a role as a phase-transfer catalyst. It is a crown ether and a saturated organic heteromonocyclic parent.
Synthesis Reference(s)
The Journal of Organic Chemistry, 39, p. 2445, 1974 DOI: 10.1021/jo00930a037
General Description
18-Crown-6 is the simplest crown ether that can be prepared by reacting triethylene glycol with triethylene glycol dichloride in the presence of potassium hydroxide as a base. 18-Crown-6 can solubilize metal salts, particularly potassium salts, in nonpolar and dipolar aprotic solvents. Thus, it is widely used as a phase transfer catalyst. It can also be used as a metal complexing agent to prepare a variety of molecular complexes.
Synthesis
A 3-l, three-necked flask equipped with a mechanical stirrer, a reflux condenser, and an addition funnel is charged with 112.5 g (100.0 ml, 0.7492 mol) of triethylene glycol and 600 ml of tetrahydrofuran. Stirring is begun and a 60% potassium hydroxide solution, prepared by dissolving 109 g (1.65 mol) of 85% potassium hydroxide in 70 ml water, is added. The solution warms slightly. After about 15 minutes of vigorous stirring (the solution begins to develop color and gradually becomes rust brown; a solution of 140.3 g (0.7503 mol) of 1,2-bis(2-chloroethoxy)ethane in 100 ml of tetrahydrofuran is added in a stream. After the addition is complete, the solution is heated at reflux and stirred vigorously for 18–24 hours. The solution is allowed to cool and the bulk of the tetrahydrofuran is evaporated under reduced pressure. The resulting thick, brown slurry is diluted with 500 ml of dichloromethane and filtered through a glass frit. The salts removed by filtration are washed with more dichloromethane to remove absorbed crown and the combined organic solution is dried over anhydrous magnesium sulfate, filtered, evaporated to minimum volume (aspirator vacuum), and distilled under high vacuum using a simple distillation head. The distillation should be carried out at the lowest possible pressure; a typical fraction contains 76–87 g (38–44%) of crude 18-crown-6 and is collected over 100–167℃ (0.2 mm) .
Purification Methods
Recrystallise it from acetonitrile and dry it in a vacuum. Purify it also by precipitating the 18-crown-6/nitromethane 1:2 complex with Et2O/nitromethane (10:1 mixture). The complex is decomposed in vacuum whereby 18-crown-6 distils off under the reduced pressure. [Beilstein 19/12 V 601.]
Properties of 18-Crown-6
| Melting point: | 42-45 °C(lit.) |
| Boiling point: | 116°C 0,2mm |
| Density | 1,175 g/cm3 |
| refractive index | 1.4580 (estimate) |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Slightly), Methanol (Very Slightly) |
| appearance | White solid |
| form | Crystals or Crystalline Mass or Liquid |
| color | White or clear colorless |
| Water Solubility | SOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,2602 |
| BRN | 1619616 |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents. |
| CAS DataBase Reference | 17455-13-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,4,7,10,13,16-Hexaoxacyclooctadecane(17455-13-9) |
| EPA Substance Registry System | 1,4,7,10,13,16-Hexaoxacyclooctadecane (17455-13-9) |
Safety information for 18-Crown-6
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for 18-Crown-6
| InChIKey | XEZNGIUYQVAUSS-UHFFFAOYSA-N |
18-Crown-6 manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 18-Crown-6-Ether pure CAS 17455-13-9View Details
18-Crown-6-Ether pure CAS 17455-13-9View Details
17455-13-9 -
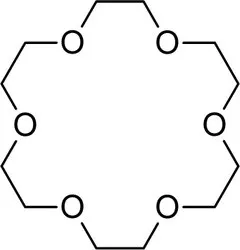 18 Crown 6 EtherView Details
18 Crown 6 EtherView Details
17455-13-9 -
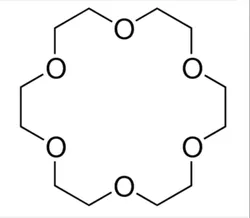 18-Crown-6-Ether (CAS Number: 17455-13-9)View Details
18-Crown-6-Ether (CAS Number: 17455-13-9)View Details
17455-13-9 -
 18-Crown-6 Ether, For LaboratoryView Details
18-Crown-6 Ether, For LaboratoryView Details
17455-13-9 -
 18-Crown-6, Grade Standard: TechnicalView Details
18-Crown-6, Grade Standard: TechnicalView Details
148-53-8 -
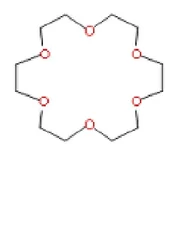 18-Crown-6View Details
18-Crown-6View Details
17455-13-9 -
 18-Crown-6-Ether CAS No: 17455-13-9View Details
18-Crown-6-Ether CAS No: 17455-13-9View Details
17455-13-9 -
 18 - CROWN - 6View Details
18 - CROWN - 6View Details
17455-13-9
