1,4-Naphthoquinone
Synonym(s):α-Naphthoquinone;1,4-Naphthalenedione, 1,4-Dihydro-1,4-diketonaphthalene;1,4-Naphthoquinone
- CAS NO.:130-15-4
- Empirical Formula: C10H6O2
- Molecular Weight: 158.15
- MDL number: MFCD00001676
- EINECS: 204-977-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 18:10:20
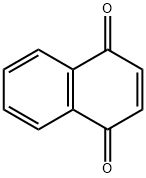
What is 1,4-Naphthoquinone?
Chemical properties
1,4-Naphthoquinone is a yellow to greenish yellow crystalline solid that has pungent odor like benzoquinone. It is slightly soluble in water, soluble in ether, chloroform, benzene, glacial acetic acid. In alkaline solutions it produces a reddish-brown color.
The Uses of 1,4-Naphthoquinone
1,4-Naphthoquinone is used as a strong dienophile in Diels-Alder reaction. It is also used as a precursor to anthroquinone and 5-nitro-1,4-naphthalenedione, which finds application in the preparation of aminoanthroquinone and is also used as a dye precursor. It is a basic component of Vitamin K.
The Uses of 1,4-Naphthoquinone
antibacterial, antineoplastic
What are the applications of Application
1,4-Naphthoquinone is a chemical studied as a potential inhibitor of MAO (monoamine oxidase) and Topo (DNA topoisomerase) activities as well as acetyltransferase activity
Definition
ChEBI: 1,4-naphthoquinone is the parent structure of the family of 1,4-naphthoquinones, in which the oxo groups of the quinone moiety are at positions 1 and 4 of the naphthalene ring. Derivatives have pharmacological properties. It derives from a hydride of a naphthalene.
What are the applications of Application
1,4-Naphthoquinone is an important organic intermediate compound, it is the chemical intermediate of fungicide dichlone and dithianon, and also the by-product of anthraquinone synthesis. It can be used as polymerisation regulator of rubber and polyester resin, synthesis of dyestuffs and drugs, algaecide, corrosion inhibitor, stabiliser of transformer oil, etc.
Origin
1,4-Naphthoquinone is a natural product that is widely distributed in nature and is found in black walnut and king walnut. Especially in several families of higher plants (like Plumbaginaceae, Juglandaceae, Ebenaceae, Boraginaceae, Dioncophyllaceae, Ancistrocladaceae, Iridaceae, Verbenaceae, Scrophulariaceae, Avicenniaceae, Balsaminaceae, Bignoniaceae, Gentianaceae, and Droseraceae), and in addition also being present in algae, fungi, some animals, and products of metabolism in some bacteria.
Synthesis Reference(s)
Synthesis, p. 330, 1977 DOI: 10.1055/s-1977-24385
Tetrahedron Letters, 31, p. 4871, 1990 DOI: 10.1016/S0040-4039(00)97755-4
General Description
Yellow needles or brownish green powder with an odor of benzoquinone.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1,4-Naphthoquinone may react with many acids and bases liberating heat and flammable gases (e.g., H2). The heat may be sufficient to start a fire in the unreacted portion of the ketone. May react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. May react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Hazard
Irritant
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition 1,4-Naphthoquinone emits toxic fumes and smoke.
Fire Hazard
Flash point data for 1,4-Naphthoquinone are not available; however, 1,4-Naphthoquinone is probably combustible.
Flammability and Explosibility
Not classified
Safety Profile
Poison by ingestion, intravenous, subcutaneous, and intraperitoneal routes. Experimental reproductive effects. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
1,4-Naphthoquinone is prepared by reacting naphthalene with molecular oxygen in a gaseous phase in the presence of a catalyst containing vanadium.
Prior to reacting the naphthalene with oxygen, the catalyst is pre-treated with molecular oxygen at 300° to 400° C in the absence of organic compounds and immediately thereafter a gas mixture containing naphthalene and molecular oxygen is passed over the pretreated catalyst at temperatures in the range of 300° to 400° C. The catalyst pre-treatment can be carried out in the presence of water vapor and the subsequent reaction of naphthalene with molecular oxygen can also be carried out in the presence of water vapor.
Potential Exposure
1,4-Naphthoquinone is used as a polymerization regulator for rubber and polyester resins; in the synthesis of dyes and pharmaceuticals; and as a fungicide and algicide.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Crystallise the quinone from diethyl ether (charcoal). It distils in steam. It also crystallises from *benzene or aqueous EtOH and sublimes in a vacuum. [Beilstein 7 IV 2422.]
Incompatibilities
Ketones are incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrated amines, azo, diazo, azido compounds, carbamates, organic cyanates. May react with many acids and bases liberating heat and flammable gases (e.g., hydrogen) generating heat may be sufficient to start a fire in the unreacted portion of the ketone. May react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (e.g., hydrogen) and heat. Incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. May react violently with aldehydes.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of 1,4-Naphthoquinone
| Melting point: | 119-122 °C(lit.) |
| Boiling point: | 243.22°C (rough estimate) |
| Density | 1,42 g/cm3 |
| vapor pressure | <1 hPa (50 °C) |
| refractive index | 1.5300 (estimate) |
| Flash point: | 141 °C |
| storage temp. | Store below +30°C. |
| solubility | 0.09g/l |
| form | Powder |
| color | Khaki |
| PH | 6.1 (10g/l, H2O, 20℃) |
| Water Solubility | insoluble |
| Merck | 14,6395 |
| BRN | 878524 |
| Stability: | Stable. Incompatible with strong reducing agents, strong oxidizing agents. |
| CAS DataBase Reference | 130-15-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,4-Naphthalenedione(130-15-4) |
| EPA Substance Registry System | 1,4-Naphthoquinone (130-15-4) |
Safety information for 1,4-Naphthoquinone
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H317:Sensitisation, Skin H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,4-Naphthoquinone
| InChIKey | FRASJONUBLZVQX-UHFFFAOYSA-N |
1,4-Naphthoquinone manufacturer
CEFA CILINAS BIOTICS PVT LTD
Suvidhinath Laboratories
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![1,4-Naphthoquinone, 5,8-dihydroxy-,,8-Dihydroxy-[1,4]naphthoquinone,5,8-dihydroxy-4-naphthoquinon](https://img.chemicalbook.in/CAS/GIF/475-38-7.gif)
You may like
-
 130-15-4 98%View Details
130-15-4 98%View Details
130-15-4 -
 1,4-Naphthoquinone CAS 130-15-4View Details
1,4-Naphthoquinone CAS 130-15-4View Details
130-15-4 -
 1,4-Naphthoquinone CAS 130-15-4View Details
1,4-Naphthoquinone CAS 130-15-4View Details
130-15-4 -
 1,4-Naphthoquinone CAS 130-15-4View Details
1,4-Naphthoquinone CAS 130-15-4View Details
130-15-4 -
 1,4-Naphthoquinone CAS 130-15-4View Details
1,4-Naphthoquinone CAS 130-15-4View Details
130-15-4 -
 1,4-Naphthoquinone CAS 130-15-4View Details
1,4-Naphthoquinone CAS 130-15-4View Details
130-15-4 -
 1,4-Naphthoquinone, For Scientific And Industrial, Grade: PureView Details
1,4-Naphthoquinone, For Scientific And Industrial, Grade: PureView Details
130-15-4 -
 1 4 NapthaquinoneView Details
1 4 NapthaquinoneView Details
130-15-4
