2,3-Dichloro-1,4-naphthoquinone
Synonym(s):2,3-Dichloro-1,4-naphthoquinone;Dichlon
- CAS NO.:117-80-6
- Empirical Formula: C10H4Cl2O2
- Molecular Weight: 227.04
- MDL number: MFCD00001677
- EINECS: 204-210-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
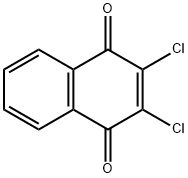
What is 2,3-Dichloro-1,4-naphthoquinone?
Chemical properties
YELLOW FINE CRYSTALLINE POWDER
The Uses of 2,3-Dichloro-1,4-naphthoquinone
Fungicide for agriculture and textiles; herbicide.
The Uses of 2,3-Dichloro-1,4-naphthoquinone
Fungicide used on fruits, field crops and vegetables
The Uses of 2,3-Dichloro-1,4-naphthoquinone
Use in textiles, as organic catalyst, as chemical intermediate, as additive in dye binders. Also used as an important catalyst to produce 3,3-dichlorobenzene.
General Description
2,3-Dichloro-1,4-naphthoquinone is a yellow crystalline solid dissolved in a water-emulsifiable liquid carrier. Can cause illness by inhalation, skin absorption and/or ingestion. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Can easily penetrate the soil and contaminate groundwater and nearby streams. Used as a fungicide.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2,3-Dichloro-1,4-naphthoquinone is a halogenated ketone. Ketones are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Health Hazard
INHALATION: Irritation to mucous membrane. EYES: Irritation. SKIN: Irritation. INGESTION: Can cause CNS depression.
Fire Hazard
Special Hazards of Combustion Products: Highly toxic fumes are imminent.
Flammability and Explosibility
Not classified
Agricultural Uses
Fungicide: Not currently registered in the U.S. Not approved for use in the EU. Dichlone is used as a fungicide for foliage and to control blue algae in ponds, swimming pools and lakes. As a substitute for copper and sulfur to control rot on fruit trees, vegetables, field crops, ornamentals, resident and commercial outdoor areas.
Trade name
ALGISTAT®; COMPOUND 604®; PHYGON®; PHYGON® PASTE; PHYGON® SEED PROTECTANT; PHYGON® XL; QUINTAR®; QUINTAR® 540F; SANQUINON®; UNIROYAL® 604; USR® 604; U.S. RUBBER® 604
Safety Profile
Poison by ingestion and intraperitoneal routes. Mildly toxic by skin contact. A skin, eye, and mucous membrane irritant. Large doses can cause central nervous system depression. Questionable carcinogen with experimental carcinogenic and neoplastigenic data. A fungcide and algicide. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORIDES.
Environmental Fate
Plant. In plants, dichlone loses both chlorine atoms and are replaced by sulphydryl
groups to give a substituted dimercapto compound (Hartley and Kidd, 1987).
Photolytic. The UV absorption band for dichlone is 330 nm (Gore et al., 1971).
Irradiation of dichlone in a variety of organic solvents (benzene, isopropanol, ethanol)
using UV light produced a number of dehalogenated compounds. In the absence or
presence of oxygen, 2-chloro-1,4-naphthoquinone, 1,4-naphthoquinone and 1,4-naphthalenediol
were produced. Further irradiation in the presence of oxygen yielded phthalic
acid and phthalic anhydride as the major products. In a mixture of benzene and isopropanol,
dichlone degraded to the minor products: 2-chloro-3-hydroxy-1,4-naphthoquinone, 2-
chloro-3-phenoxy-1,4-naphthoquinone, 2,3-dichloro-4-hydroxy-1-keto-2-phenyl-1,2-
dihydronaphthalene and isopropyl-1-chloro-2,3-dioxo-1-indanecarboxylate (Ide et al.,
1979).
Chemical/Physical. Emits toxic fumes of chlorine when heated to decomposition (Sax
and Lewis, 1987).
Purification Methods
Crystallise the quinone from EtOH. [Beilstein 7 IV 2426.]
Properties of 2,3-Dichloro-1,4-naphthoquinone
| Melting point: | 194-197 °C (lit.) |
| Boiling point: | 275 °C (2 mmHg) |
| Density | 1.4057 (rough estimate) |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1.5410 (estimate) |
| Flash point: | 275°C/2mm |
| storage temp. | Store below +30°C. |
| form | Fine Crystalline Powder |
| color | Yellow |
| Water Solubility | 0.008 g/L |
| Merck | 14,3045 |
| BRN | 1073511 |
| CAS DataBase Reference | 117-80-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,4-Naphthalenedione, 2,3-dichloro-(117-80-6) |
| EPA Substance Registry System | Dichlone (117-80-6) |
Safety information for 2,3-Dichloro-1,4-naphthoquinone
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,3-Dichloro-1,4-naphthoquinone
2,3-Dichloro-1,4-naphthoquinone manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran





You may like
-
 DICHLONE 98%View Details
DICHLONE 98%View Details -
 Dichlone 98%View Details
Dichlone 98%View Details -
 117-80-6 99%View Details
117-80-6 99%View Details
117-80-6 -
 Dichlone 98%View Details
Dichlone 98%View Details
117-80-6 -
 Dichlone 117-80-6 98%View Details
Dichlone 117-80-6 98%View Details
117-80-6 -
 2,3-Dichloro-1,4-naphthoquinone CAS 117-80-6View Details
2,3-Dichloro-1,4-naphthoquinone CAS 117-80-6View Details
117-80-6 -
 2,3 Dichloro 1,4-naphthoquinone, 98% CAS 117-80-6View Details
2,3 Dichloro 1,4-naphthoquinone, 98% CAS 117-80-6View Details
117-80-6 -
 2,3-Dichloronaphthalene-1,4-Dione (Dichlone)View Details
2,3-Dichloronaphthalene-1,4-Dione (Dichlone)View Details
117-80-6
