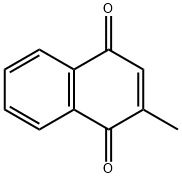
2-Hydroxy-1,4-naphoquinone
Synonym(s):Lawsone;Natural Orange 6
- CAS NO.:83-72-7
- Empirical Formula: C10H6O3
- Molecular Weight: 174.15
- MDL number: MFCD00001678
- EINECS: 201-496-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
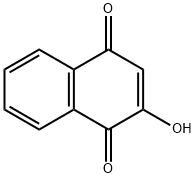
What is 2-Hydroxy-1,4-naphoquinone?
Chemical properties
Yellow crystal powder
History
Lawsone (CI Natural Orange 6; CI 75420), also known as henna and isojuglone, occurs in the shrub henna (Lawsone alba). In England, the plant is known as Egyptian privet. The dye was extracted from the leaves of the plant, using sodium bicarbonate, and the extracts used to dye protein fibers an orange shade. Henna is probably the oldest cosmetic known. The ancient Egyptians used it as a hair dye and for staining fingernails. It is said that Mohammed dyed his beard with henna. Lawsone has been identified as 2-hydroxy-1,4-naphthoquinone. It has been synthesized by the Thiele acetylation of 1,4-naphthoquinone followed by hydrolysis and oxidation.
The Uses of 2-Hydroxy-1,4-naphoquinone
antifungal, sunscreen, antibacterial, antineoplastic
The Uses of 2-Hydroxy-1,4-naphoquinone
2-Hydroxy-1,4-naphthoquinone is used for preparing decorative hair and skin dyes. 2-Hydroxy-1,4-naphthoquinone also demonstrates antimicrobial and antioxidant effects. It also suppress the formation of hydrogen peroxide and superoxide radical anion by aldehyde oxidase-catalyzed reactions.
Definition
A coloring principle obtained from dried leaves of certain tropical plants (North Africa, India).
Synthesis Reference(s)
The Journal of Organic Chemistry, 53, p. 808, 1988 DOI: 10.1021/jo00239a023
Tetrahedron Letters, 25, p. 533, 1984 DOI: 10.1016/S0040-4039(00)99930-1
General Description
Yellow prisms or yellow powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Phenols, such as 2-Hydroxy-1,4-naphoquinone, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid. Nitrated phenols often explode when heated. Many of them form metal salts that tend toward detonation by rather mild shock.
Health Hazard
ACUTE/CHRONIC HAZARDS: 2-Hydroxy-1,4-naphoquinone may be absorbed through the skin and can cause skin irritation.
Fire Hazard
Flash point data for 2-Hydroxy-1,4-naphoquinone are not available but 2-Hydroxy-1,4-naphoquinone is probably combustible.
Contact allergens
Henna, prepared by powdering the dried leaves of henna plant (Lawsonia inermis L.), is used for coloring and conditioning hair and nails, particularly by Muslims or Hindus. It contains Lawsone, which very rarely induces contact allergy. Most dermatitis caused by “black henna” is due to PPD and derivatives
Purification Methods
Crystallise Lawsone B from *C6H6 or AcOH (m 192.5o, 195-196o). It sublimes in a vacuum (m 194o). It has UV with max at 455nm (aqueous NaOH). [Beilstein 8 H 300, 8 I 635, 8 II 344, 8 III 2543, 8 IV 2360.]
Properties of 2-Hydroxy-1,4-naphoquinone
| Melting point: | 192-195 °C (dec.)(lit.) |
| Boiling point: | 265.11°C (rough estimate) |
| Density | 1.2346 (rough estimate) |
| refractive index | 1.5036 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Acetonitrile (Slightly), Chloroform (Slightly), Methanol (Slightly) |
| form | Crystalline Powder |
| pka | 4.31±0.10(Predicted) |
| Colour Index | 75480 |
| color | Yellow |
| Water Solubility | 2 g/L (20 ºC) |
| Merck | 14,5393 |
| BRN | 1565260 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 83-72-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,4-Naphthalenedione, 2-hydroxy-(83-72-7) |
| EPA Substance Registry System | 2-Hydroxy-1,4-naphthoquinone (83-72-7) |
Safety information for 2-Hydroxy-1,4-naphoquinone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Hydroxy-1,4-naphoquinone
| InChIKey | CSFWPUWCSPOLJW-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 83-72-7 2-hydroxy-1,4-napthoquinone 98%View Details
83-72-7 2-hydroxy-1,4-napthoquinone 98%View Details
83-72-7 -
 2-hydroxynaphthalene-1,4-dione 98%View Details
2-hydroxynaphthalene-1,4-dione 98%View Details
83-72-7 -
 2-hydroxynaphthalene1,4-dione 83-72-7 98%View Details
2-hydroxynaphthalene1,4-dione 83-72-7 98%View Details
83-72-7 -
 83-72-7 2 - hydroxynaphthalene - 1,4 - dione ( Lawsone ) 98%View Details
83-72-7 2 - hydroxynaphthalene - 1,4 - dione ( Lawsone ) 98%View Details
83-72-7 -
 2-Hydroxy-1,4-naphthoquinone CAS 83-72-7View Details
2-Hydroxy-1,4-naphthoquinone CAS 83-72-7View Details
83-72-7 -
 2-Hydroxy-1,4-naphthoquinone, 98% CAS 83-72-7View Details
2-Hydroxy-1,4-naphthoquinone, 98% CAS 83-72-7View Details
83-72-7 -
 2-Hydroxy-1,4-naphthoquinone CAS 83-72-7View Details
2-Hydroxy-1,4-naphthoquinone CAS 83-72-7View Details
83-72-7 -
 2-Hydroxy-1,4-naphthoquinone CAS 83-72-7View Details
2-Hydroxy-1,4-naphthoquinone CAS 83-72-7View Details
83-72-7
