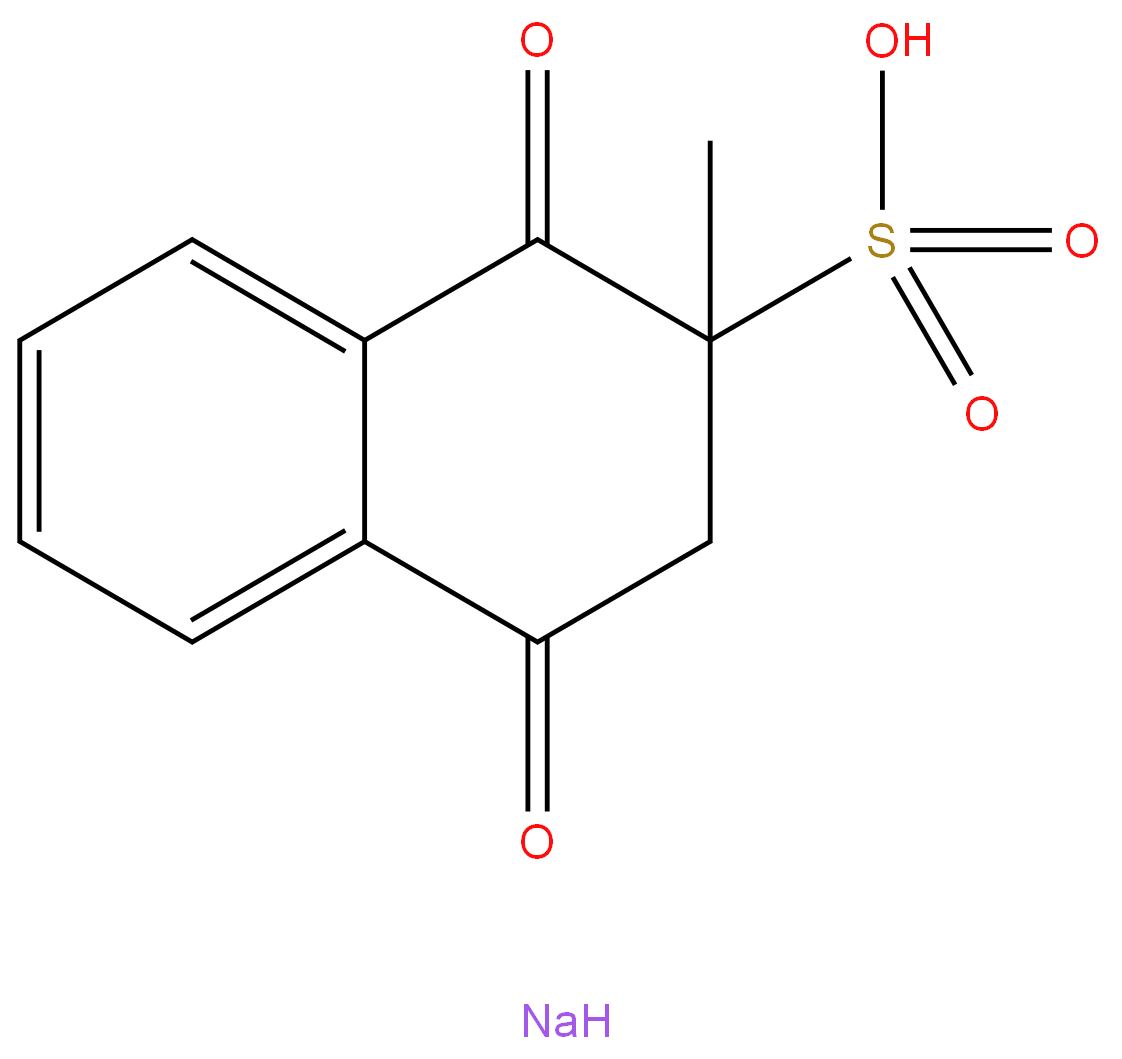
Menadione sodium bisulfite
Synonym(s):2-Methyl-1,4-naphthoquinone sodium bisulfite
- CAS NO.:130-37-0
- Empirical Formula: C11H11NaO5S
- Molecular Weight: 278.25
- MDL number: MFCD08063339
- EINECS: 204-987-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
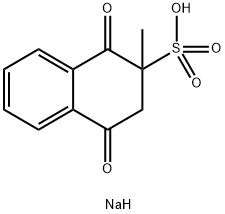
What is Menadione sodium bisulfite?
Description
Menadione sodium bisulfite (MSB, also known as vitamin K3), is a crucial growth regulator mediating plant defense response. This substance is used clinically in China to treat hemorrhagic diseases caused by vitamin K deficiency and globally as a vitamin K supplement. This compound and its complex (MSBC) are water-soluble compounds. Menadione and the menadione sodium bisulfite complex are the sources of vitamin K activity in commercial laboratory rodent diets. Menadione possesses high biological activity, but its absorption depends on the amount of fat in the diet. Compared with Menadione, the menadione sodium bisulfite complex is more readily absorbed and is almost as active on a molar basis as phylloquinone. XTT, a tetrazolium salt, could converted to formazan in the presence of an electron coupling reagent such as menadione sodium bisulfite. The formed formazan does not need to be solubilized. Hence, the XTT test, a cytotoxicity evaluation method, is quicker and safer compared with MTT[1-2].
Chemical properties
white powder
The Uses of Menadione sodium bisulfite
Menadione Sodium Bisulfite may be used as a safe and suitable source of Vitamin K activity in the food for all animals in the United States in accordance with good manufacturing and feeding practices.
Originator
Kavitol,Lannacher Heilmittel
The Uses of Menadione sodium bisulfite
Menadione sodium bisulfite has been used:
- as a priming agent in tomato for improving salt tolerance
- to induce reactive oxygen species (ROS) in human lung epithelium BEAS-2B microtissues
- to induce endogenous superoxide production in mouse C17.2 neural stem cells
Definition
ChEBI: Menadione sodium sulfonate is an organic sodium salt that is the monosodium salt of menadione sulfonate. A synthetic naphthoquinone without the isoprenoid side chain and biological activity, but can be converted into active vitamin K2, menaquinone, after alkylation in vivo. It contains a menadione sulfonate. It is functionally related to a menadione.
Manufacturing Process
The 2-naphthalenesulfonic acid, 1,2,3,4-tetrahydro-2-methyl-1,4-dioxo-, sodium salt, trihydrate can be prepared by mixing the 2-methyl-1,4- naphthoquinone with the bisulphite salt in the presence of water. Ordinarily gentle warming of the aqueous mixture is preferred to facilitate solution. The mixture of 2-methyl-1,4-naphthoquinone (250 mg; 1 molar equivalent); sodium bisulphite (149 mg; 1 molar equivalent); distilled water (250 ml) or 2- methyl-1,4-naphthoquinone (250 mg; 1 molar equivalent); potassium bisulphate (349 mg; 2 molar equivalent); distilled water 250 ml may be used. These examples representing preferred ratios of ingredients are merely illustrative and are not to be interpreted as limiting.
The bisulphite addition compounds have been found to be stable in sunlight
and also to be heat stable. Tests, for example, carried out in ampoules have
shown aqueous solutions of the compounds not to be decomposed after
exposure to a month's sunlight, while other tests have shown the solutions of such compounds to retain their original potency (a) when stored in an oven at
60°C for 15 days or (b) when sterilized at 15 pounds for 0.5 hour in an
autoclave at about 122°C. These properties emphasize the radical differences
between the stable salts and the properties of 2-methyl-1,4-naphthoquinone,
the characteristic instability of which is illustrated by its sensitivity, i. e.,
decomposition, when exposed to light.
The bisulphite addition compounds have a vitamin K activity equal to that of
the 2-methyl-1,4-naphthoquinone contained in the molecule. The compounds,
although suitable for oral administration, are particularly adaptable in aqueous
solution for parenteral administration in the treatment of hemorrhagic
conditions.
brand name
Hykinone (Abbott); Klotogen (Abbott).
Therapeutic Function
Prothrombogenic vitamin
Biochem/physiol Actions
Menadione sodium bisulfite is a water-soluble form of menadione, which belongs to the Vitamin K class of compounds. These are necessary for the biosynthesis of prothrombin and other blood clotting factors. Menadione is a prothrombogenic compound and is used as a model quinone in cell culture and in vivo investigations. Menadione has been shown to affect gap-junctional intercellular communication by mediation of tyrosine phosphorylation. Menadione has demonstrated cytotoxic activity against a variety of cell lines and can induce apoptosis in cultured cells, such as osteoclasts and osteoblasts, via elevation of peroxide and superoxide radical levels.An HPLC method for detection of menadione sodium bisulfite in multivitamin formulations has been published. A chemiluminescence assay for menadione sodium bisulfite in pharmaceutical preparations and biological fluids has been reported.
Side Effects
Inflammation of the walls of a vein
Facial flushing
Cardiovascular collapse
Severe shock-like reactions
Sweating
Injection site reactions
Chest constriction
Pain
Shortness of breath
Bluish discoloration of the skin
References
[1] Zhang Y, et al. Menadione sodium bisulfite inhibits the toxic aggregation of amyloid-β(1-42). Biochimica et Biophysica Acta (BBA) - General Subjects, 2018; 1862: 2226-2235.
[2] Lustgarten M, et al. An Objective Appraisal of the Free Radical Theory of Aging. Handbook of the Biology of Aging, 2011; 177-202.
Properties of Menadione sodium bisulfite
| Melting point: | 121-124°C |
| storage temp. | -20°C |
| solubility | H2O: ≥50mg/mL, clear, colorless to faintly yellow |
| form | Solid |
| color | White to off-white |
| InChI | InChI=1S/C11H10O5S.Na.H/c1-11(17(14,15)16)6-9(12)7-4-2-3-5-8(7)10(11)13;;/h2-5H,6H2,1H3,(H,14,15,16);; |
| EPA Substance Registry System | Menadione sodium bisulfite (130-37-0) |
Safety information for Menadione sodium bisulfite
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
Computed Descriptors for Menadione sodium bisulfite
| InChIKey | JWNUEPMMANLFQO-UHFFFAOYSA-N |
| SMILES | O=C1C(CC(=O)C2=CC=CC=C12)(C)S(O)(=O)=O.[NaH] |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Menadione sodium bisulfite 99%View Details
Menadione sodium bisulfite 99%View Details -
 Menadione sodium bisulfite 98%View Details
Menadione sodium bisulfite 98%View Details -
 Menadione sodium bisulfite 98% CAS 130-37-0View Details
Menadione sodium bisulfite 98% CAS 130-37-0View Details
130-37-0 -
 Menadione sodium bisulfite 98% (HPLC) CAS 130-37-0View Details
Menadione sodium bisulfite 98% (HPLC) CAS 130-37-0View Details
130-37-0 -
 Menadione sodium bisulphite CAS 130-37-0View Details
Menadione sodium bisulphite CAS 130-37-0View Details
130-37-0 -
 MENADIONE SODIUM BISULPHITE Pure CAS 130-37-0View Details
MENADIONE SODIUM BISULPHITE Pure CAS 130-37-0View Details
130-37-0 -
 Menadione sodium bisulfite CAS 130-37-0View Details
Menadione sodium bisulfite CAS 130-37-0View Details
130-37-0 -
 Menadione sodium bisulfite CAS 130-37-0View Details
Menadione sodium bisulfite CAS 130-37-0View Details
130-37-0
