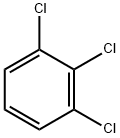
1,2,3-Trichlorobenzene
- CAS NO.:87-61-6
- Empirical Formula: C6H3Cl3
- Molecular Weight: 181.45
- MDL number: MFCD00000537
- EINECS: 201-757-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
What is 1,2,3-Trichlorobenzene?
Description
Trichlorobenzenes (TCBs) are synthetic chemicals that occur in three different isomeric forms. The three chlorinated cyclic aromatic isomers are 1,2,3-trichlorobenzene (1,2,3-TCB), 1,2,4-trichlorobenzene (1,2,4-TCB), and 1,3,5-trichlorobenzene (1,3,5-TCB). 1,2,4-TCB is one of the 188 chemicals designated as a hazardous air pollutant under the Clean Air Act.
Chemical properties
1,2,3-Trichlorobenzene and 1,3,5-trichlorobenzene are colorless solids, while 1,2,4-trichlorobenzene is a colorless liquid. Although the three isomers of trichlorobenzenes have the same molecular weight and formula, they each may have different chemical and toxicological properties. One of the isomers (1,2,4-trichlorobenzene) is produced in large quantities and is used as a solvent to dissolve such special materials as oils, waxes, resins, greases, and rubber. It is also frequently used to produce dyes and textiles. The other two isomers, 1,2,3-trichlorobenzene and 1,3,5-trichlorobenzene, are produced in lower quantities and have fewer uses.
The Uses of 1,2,3-Trichlorobenzene
Detoxification by catalytic hydrotreatment of 1,2,3-Trichlorobenzene is used for the disposal of hazardous organic waste liquids. As constituent of trichlorobenzene mixt used for termite control. As transformer fluid, dye carrier & solvent. Solvent for high melting products, Coolant in electrical installations and glass tempering. In polyester dyeing, lubricants, Heat transfer medium. As chemical intermediate for 2,3-dichlorophenol.
The Uses of 1,2,3-Trichlorobenzene
Trichlorobenzenes are primarily used as solvents in chemical manufacturing industries. 1,2,4-Trichlorobenzene is economically the most important isomer. 1,2,4-Trichlorobenzene is used as a solvent in chemical reactions to dissolve oils, waxes, and resins. Furthermore, it is also used as a dye carrier. 1,2,3- Trichlorobenzene is used as an intermediate for pesticides production, pigments, and dyes. 1,3,5-Trichlorobenzene is not marketed commercially and has very limited use as a chemical intermediate. Besides, trichlorobenzenes can also be used as degreasing agents, as septic tanks and drain cleaners, and as an ingredient in wood preservatives and abrasive formulations. Other minor uses include metal work, anticorrosive paint, and corrosion inhibitor in sprays. In the past, mixed isomers of trichlorobenzenes were used to control termites; however, their use has been discontinued.
What are the applications of Application
1,2,3-Trichlorobenzene is an isomer of 1,2,4-Trichlorobenzene
Definition
ChEBI: 1,2,3-trichlorobenzene is a trichlorobenzene carrying chloro substituents at positions 1, 2 and 3.
General Description
A white solid with a sharp chlorobenzene odor. Insoluble in water and denser than water. Hence sinks in water. Melting point 63-64°C (145-147°F).
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1,2,3-Trichlorobenzene can react with oxidizing agents. . May emit toxic hydrogen chloride and phosgene gases in fire.
Health Hazard
Inhalation may cause irritation of respiratory tract. Irritating to the eyes. May redden skin on contact. Ingestion may cause liver damage.
Environmental Fate
Biological. Under aerobic conditions, soil microbes are capable of degrading 1,2,3-
trichlorobenzene to 1,2- and 1,3-dichlorobenzene and carbon dioxide (Kobayashi and Rittman,
1982). A mixed culture of soil bacteria or a Pseudomonas sp. transformed 1,2,3-trichlorobenzene
to 2,3,4-, 3,4,5-, and 2,3,6-trichlorophenol (Ballschiter and Scholz, 1980).
In an enrichment culture derived from a contaminated site in Bayou d’Inde, LA, 1,2,3-
trichlorobenzene underwent reductive dechlorination to 1,2- and 1,3-dichlorobenzene at relative
molar yields of 1 and 99%, respectively. The maximum dechlorination rate, based on the
recommended Michaelis-Menten model, was 60 nM/d (Pavlostathis and Prytula, 2000).
Photolytic. The sunlight irradiation of 1,2,3-trichlorobenzene (20 g) in a 100-mL borosilicate
glass-stoppered Erlenmeyer flask for 56 d yielded 32 ppm pentachlorobiphenyl (Uyeta et al.,
1976).
Chemical/Physical. At 70.0 °C and pH values of 3.07, 7.13, and 9.80, the hydrolysis half-lives
were calculated to be 19.2, 15.0, and 34.4 d, respectively (Ellington et al., 1986).
Emits toxic chloride fumes when heated to decomposition.
Purification Methods
Crystallise it from EtOH. [Beilstein 5 IV 664.]
Toxicity evaluation
The liver is themain target of trichlorobenzenes irrespective of the route of exposure. The mechanisms of liver toxicity induced by these chemicals have not been illustrated. It might involve intermediate arene oxides formed during initial transformation to trichlorophenols. In addition, exposure to 1,2,4-TCB induced porphyria in rats by inducing δ-aminolevulinic acid (ALA) synthetase, a rate-limiting enzyme in the biosynthesis of heme, and also heme oxygenase, a ratelimiting enzyme in the degradation of heme synthetase, and therefore increasing heme production.
Properties of 1,2,3-Trichlorobenzene
| Melting point: | 51-53 °C (lit.) |
| Boiling point: | 218-219 °C (lit.) |
| Density | 1,69 g/cm3 |
| vapor density | 6.25 (vs air) |
| vapor pressure | 0.07 mm Hg ( 25 °C) |
| refractive index | 1.5693 (estimate) |
| Flash point: | 260 °F |
| storage temp. | 2-8°C |
| solubility | 0.02g/l |
| form | neat |
| color | White crystals or platelets |
| explosive limit | 2.5-6.6%(V) |
| Water Solubility | INSOLUBLE |
| Merck | 14,9630 |
| BRN | 956882 |
| Henry's Law Constant | 8.36, 10.3, 13.2, 20.6, and 26.5 at 2.0, 6.0, 10.0, 18.0, and 25.0 °C, respectively (EPICS-SPME,
Dewulf et al., 1999) |
| CAS DataBase Reference | 87-61-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1,2,3-trichloro-(87-61-6) |
| EPA Substance Registry System | 1,2,3-Trichlorobenzene (87-61-6) |
Safety information for 1,2,3-Trichlorobenzene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for 1,2,3-Trichlorobenzene
1,2,3-Trichlorobenzene manufacturer
JSK Chemicals
Panoli Intermediates (India) Pvt., Limited. (Kutch Chemical Industries Ltd.)
Aarti Industries Limited (AIL)
LANXESS India Pvt. Ltd.
Hema Dye Chem Pvt Ltd
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 1,2,3-Trichlorobenzene 98%View Details
1,2,3-Trichlorobenzene 98%View Details -
 1,2,3-Trichlorobenzene 87-61-6 98%View Details
1,2,3-Trichlorobenzene 87-61-6 98%View Details
87-61-6 -
 1,2,3-Trichlorobenzene CAS 87-61-6View Details
1,2,3-Trichlorobenzene CAS 87-61-6View Details
87-61-6 -
 87-61-6 1,2,3-Trichlorobenzene 98%View Details
87-61-6 1,2,3-Trichlorobenzene 98%View Details
87-61-6 -
 1,2,3-Trichlorobenzene 87-61-6 98%View Details
1,2,3-Trichlorobenzene 87-61-6 98%View Details
87-61-6 -
 1,2,3-Trichlorobenzene 99%View Details
1,2,3-Trichlorobenzene 99%View Details
87-61-6 -
 1,2,3-Trichlorobenzene CAS 87-61-6View Details
1,2,3-Trichlorobenzene CAS 87-61-6View Details
87-61-6 -
 1,2,3-Trichlorobenzene CAS 87-61-6View Details
1,2,3-Trichlorobenzene CAS 87-61-6View Details
87-61-6