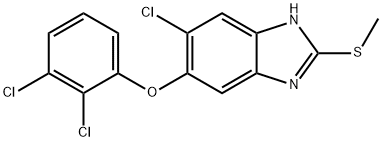
Triclabendazole
Synonym(s):5-Chloro-6-(2,3-dichlorophenoxy)-2-(methylthio)-1H-benzimidazole;Triclabendazole
- CAS NO.:68786-66-3
- Empirical Formula: C14H9Cl3N2OS
- Molecular Weight: 359.66
- MDL number: MFCD00864519
- EINECS: 614-728-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
What is Triclabendazole?
Absorption
After a single oral dose of 10 mg/kg triclabendazole with a 560-kcal meal to patients diagnosed with fascioliasis, mean peak plasma concentrations (Cmax) for triclabendazole, the sulfoxide, and sulfone metabolites were 1.16, 38.6, and 2.29 μmol/L, respectively. The area under the curve (AUC) for triclabendazole, the sulfoxide and sulfone metabolites were 5.72, 386, and 30.5 μmol?h/L, respectively.
After the oral administration of a single dose of triclabendazole at 10 mg/kg with a 560 calorie meal to patients with fascioliasis, the median Tmax for the parent compound as well as the active sulfoxide metabolite was 3 to 4 hours.
Effect of Food
Cmax and AUC of triclabendazole and sulfoxide metabolite increased about 2-3 times when triclabendazole was administered as a single dose at 10 mg/kg with a meal containing approximately 560 calories. Additionally, the sulfoxide metabolite Tmax increased from 2 hours in fasting subjects to 4 hours in fed subjects .
Toxicity
Oral LD50 (rat): >8 gm/kg; Oral LD50 (mouse): >8 gm/kg
A note on the use in pregnancy
There are no available data on triclabendazole use in pregnant women to calculate a drug associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Reproductive studies in animals (rat and rabbits) have not demonstrated an increased risk of increased fetal abnormalities with exposure to triclabendazole during the organogenesis period at doses which were about 0.3 to 1.6 times the maximum recommended human dose (MRHD) of 20 mg/kg.
Carcinogenesis/Mutagenesis
No genotoxic risk was noted for triclabendazole tested in 6 genotoxicity in vitro and in vivo assays.
Impairment of Fertility
No drug-related effects on reproductive performance, mating ratios or indices of fertility have been observed in a 2-generation reproductive and developmental toxicity study in rats.
A note on use in breastfeeding
There are no human findings on the presence of triclabendazole in milk, the effects on a nursing infant, or the effects on maternal milk production. The results of animal studies indicate that triclabendazole is found in goat milk when given as a single dose to a lactating female goat. When a drug is found to be present in animal milk, the likelihood that it will be found in human milk is high. Excercise caution if this drug is administered during nursing.
Description
Triclabendazole is a benzimidazole anthelmintic. It eliminates both immature and adult flukes in a sheep model of F. hepatica infection when administered at a dose of 10 mg/kg. Triclabendazole (5 μM) also protects against glucose- or α-synuclein-induced apoptosis in S. cerevisiae via inhibition of adenylyl cyclase in the Ras-adenylyl cyclase-protein kinase A (PKA) nutrient sensing pathway.
Chemical properties
Off-White Solid
The Uses of Triclabendazole
Triclabendazole is classified under the category of ‘Anthelmintic flukicide for veterinary use.
The Uses of Triclabendazole
An anthelmintic (fasciola).
Background
Triclabendazole, manufactured by Novartis pharmaceuticals, is an antihelminthic drug that was approved by the FDA in February 2019 for the treatment of fascioliasis in humans. Fascioliasis is a parasitic infection often caused by the helminth, Fasciola hepatica, which is also known as “the common liver fluke” or “the sheep liver fluke” or by Fasciola gigantica, another helminth. These parasites can infect humans following ingestion of larvae in contaminated water or food.
Triclabendazole was previously used in the treatment of fascioliasis in livestock, but is now approved for human use.
This drug is currently the only FDA-approved drug for individuals with fascioliasis, which affects 2.4 million people worldwide.
Indications
This drug is indicated for the treatment of fascioliasis in patients aged 6 years old and above.
What are the applications of Application
Triclabendazole is an anthelminitic
Definition
ChEBI: 6-chloro-5-(2,3-dichlorophenoxy)-2-(methylthio)-1H-benzimidazole is an aromatic ether.
Pharmacokinetics
Triclabendazole and its metabolites are active against both the immature and mature worms of Fasciola hepatica and
Fasciola gigantica helminths.
Effect on QT interval
This drug may prolong the cardiac QT interval. Monitor ECG in patients with a history of QT prolongation or who are taking medications known to prolong the QT interval.
Metabolism
Based on in vitro studies, triclabendazole is mainly metabolized by CYP1A2 enzyme (approximately 64%) into its active sulfoxide metabolite and to a lesser extent by CYP2C9, CYP2C19, CYP2D6, CYP3A, and FMO (flavin containing monooxygenase). This sulfoxide metabolite is further metabolized mainly by CYP2C9 to the active sulfone metabolite, and to a smaller extent by CYP1A1, CYP1A2, CYP1B1, CYP2C19, CYP2D6, and CYP3A4, in vitro.
Properties of Triclabendazole
| Melting point: | 175-176°C |
| Boiling point: | 240.6°C (rough estimate) |
| Density | 1.3875 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Acetonitrile (Slightly, Sonicated), Methanol (Slightly) |
| form | neat |
| pka | 7.91±0.10(Predicted) |
| form | Solid |
| color | White to Beige |
| Merck | 14,9652 |
| Stability: | Stability |
| InChI | InChI=1S/C14H9Cl3N2OS/c1-21-14-18-9-5-8(16)12(6-10(9)19-14)20-11-4-2-3-7(15)13(11)17/h2-6H,1H3,(H,18,19) |
| CAS DataBase Reference | 68786-66-3(CAS DataBase Reference) |
Safety information for Triclabendazole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Triclabendazole
| InChIKey | NQPDXQQQCQDHHW-UHFFFAOYSA-N |
| SMILES | C1(SC)NC2=CC(Cl)=C(OC3=CC=CC(Cl)=C3Cl)C=C2N=1 |
Triclabendazole manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Triclabendazole 99%View Details
Triclabendazole 99%View Details -
 TRICLABENDAZOLE 99%View Details
TRICLABENDAZOLE 99%View Details -
 TRICLABENDAZOLE 99%View Details
TRICLABENDAZOLE 99%View Details -
 68786-66-3 Triclabendazole 99%View Details
68786-66-3 Triclabendazole 99%View Details
68786-66-3 -
 Triclabendazole 95-99 %View Details
Triclabendazole 95-99 %View Details
68786-66-3 -
 Triclabendazole 95% CAS 68786-66-3View Details
Triclabendazole 95% CAS 68786-66-3View Details
68786-66-3 -
 Triclabendazole CAS 68786-66-3View Details
Triclabendazole CAS 68786-66-3View Details
68786-66-3 -
 Triclabendazole drug intermediateView Details
Triclabendazole drug intermediateView Details
118353-04-1
