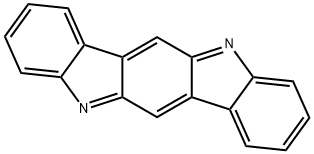
11,12-Dihydroindolo[2,3-a]carbazole
- CAS NO.:60511-85-5
- Empirical Formula: C18H12N2
- Molecular Weight: 256.3
- MDL number: MFCD07371389
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-19 18:29:52
![11,12-Dihydroindolo[2,3-a]carbazole Structural](https://img.chemicalbook.in/CAS/GIF/60511-85-5.gif)
What is 11,12-Dihydroindolo[2,3-a]carbazole?
Chemical properties
Flash Point:269.9±13.9 °C
The Uses of 11,12-Dihydroindolo[2,3-a]carbazole
11,12-Dihydroindolo[2,3-a]carbazole is an isomer of indole[3,2-b]carbazole. 11,12-Dihydroindolo[2,3-a]carbazole derivatives have good planarity, high thermal stability, and excellent photophysical properties. As an optoelectronic material, it is often used to synthesize TADF-OLED body and emission layer materials and as a pharmaceutical intermediate to synthesize anticancer drugs[1-3]. Chemical sensors based on indole[2,3-a]carbazole can be used to detect Cu2+ and Fe3+ in environmental and biological systems simultaneously[4]. Compared to other metal ions, the sensor has specificity and high sensitivity for the detection of Cu2+ and Fe3+ ions. For Cu2+ and Fe3+ ions, detection limits are as low as 2.93×10 -7 M and 4.10×10 -7 M.
Preparation
1,2-cyclohexanedione (1 g, 8.93 mmol) and phenylhydrazine hydrochloride (3.86 g, 26.78 mmol) were added to the reactor. Nitrogen is flushed into the flask, followed by 20 mL of acetic acid. After stirring and refluxing for 24 hours, the mixture is cooled to room temperature. The reaction mixture is slowly poured into ice water and stirred to precipitate a large amount of yellow solids. It is then filtered, washed with pure water, and dried. After purification, 11,12-Dihydroindolo[2,3-a]carbazole was obtained. Yield=95.6%![11,12-DIHYRDOINDOLO[2,3-A]CARBAZOLE 11,12-DIHYRDOINDOLO[2,3-A]CARBAZOLE](/NewsImg/2023-09-11/6383003895736949039297168.jpg)
Benefits
11,12-Dihydroindolo[2,3-a]carbazole could used as a donor unit. The electron-rich carbazole and indole units are contained in the plane donor, which helps to increase the electron-donating ability and hole-transporting ability.
References
[1] Tian J, et al. Synthesis and thermal, electrochemical, photophysical properties of novel symmetric/asymmetric indolo[2,3-a]carbazole derivatives bearing different aryl substituents. Tetrahedron, 2016; 73: 230-238.
[2] Zenkov R, et al. Indolo[2,3- a ]carbazoles: diversity, biological properties, application in antitumor therapy. Chemistry of Heterocyclic Compounds, 2020; 56: 644–658.
[3] Yang S, et al. Synthesis and characterization of novel butterfly-shaped aryl-substituted indolo[2,3-a]carbazole derivatives. Tetrahedron Letters, 2015; 56: 2223-2227.
[4] Chen ;Z, et al. A simple indolo[2,3-a]carbazole based colorimetric chemosensor for simultaneous detection of Cu2+ and Fe3+ ions. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2020; 234: 118236.
Properties of 11,12-Dihydroindolo[2,3-a]carbazole
| Melting point: | 371°C(lit.) |
| Boiling point: | 569.8±23.0 °C(Predicted) |
| Density | 1.404±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| form | powder to crystal |
| pka | 16.70±0.30(Predicted) |
| color | White to Light yellow |
| λmax | 357nm(EtOH)(lit.) |
| InChI | InChI=1S/C18H12N2/c1-3-7-15-11(5-1)13-9-10-14-12-6-2-4-8-16(12)20-18(14)17(13)19-15/h1-10,19-20H |
Safety information for 11,12-Dihydroindolo[2,3-a]carbazole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 11,12-Dihydroindolo[2,3-a]carbazole
| InChIKey | NSNKTSSANPWFJA-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=CC=C2)C2=C1C1NC3=C(C=1C=C2)C=CC=C3 |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![Indolo[3,2-b]carbazole](https://img.chemicalbook.in/CAS/GIF/6336-32-9.gif)
You may like
-
![11,12-Dihydroindolo[2,3-a]carbazole CAS 60511-85-5](https://img.chemicalbook.in//Content/image/CP5.jpg) 11,12-Dihydroindolo[2,3-a]carbazole CAS 60511-85-5View Details
11,12-Dihydroindolo[2,3-a]carbazole CAS 60511-85-5View Details
60511-85-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1