Indole-3-carbinol
Synonym(s):3-Indolemethanol
- CAS NO.:700-06-1
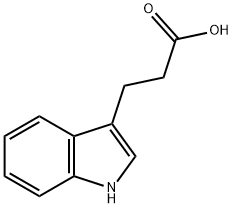
- Empirical Formula: C9H9NO
- Molecular Weight: 147.17
- MDL number: MFCD02683932
- EINECS: 211-836-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
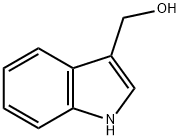
What is Indole-3-carbinol?
Chemical properties
Indole-3-carbinol is an off-white powder. It is soluble in organic solvents such as benzene, ethanol, DMSO, and dimethyl formamide (DMF), which should be purged with an inert gas. The solubility of Indole-3-carbinol in ethanol and DMF is approximately 10 mg/ml and approximately 3 mg/ml in DMSO. Indole-3-carbinol is an unstable compound that undergoes rapid oligomerization in acid pH environments, like the stomach.
Occurrence
Indole-3-carbinol is produced by members of the family Cruciferae, and particularly members of the genus Brassica (e.g., cabbage, radishes, cauliflower, broccoli, Brussels sprouts, and daikon).
The Uses of Indole-3-carbinol
Indole-3-carbinol has been used for encapsulation with poly-lactic-co-glycolic acid (PLGA), to study its in-vitro anti-cancerogenic effects on breast adenocarcinoma epithelial (MCF7), colon adenocarcinoma epithelial (Caco2), prostate carcinoma epithelial (PC3) cells. It has also been used as a cytochrome P4501A (CYP1A) inducer.
What are the applications of Application
Indole-3-carbinol is a cell growth inhibitor
Definition
ChEBI: Indole-3-carbinol is an indolyl alcohol carrying a hydroxymethyl group at position 3. It is a constituent of the cruciferous vegetables and had anticancer activity.
What are the applications of Application
Indole-3-carbinol (I3C) is a secondary plant metabolite of 3-methylindole produced in Brassica family vegetables. It is an inhibitor of Amyloid-beta deposition and Inhibits cancinogenesis at the initiation stage. Indole-3-carbinol is used in cancer prevention, specifically for breast, cervical and colon cancers. It has also been widely used to address lupus.
Preparation
Using indole, phosphorus oxychloride and N,N-dimethylformamide as raw materials, indole-3-carbaldehyde was synthesized by Vilsmeier-Haack reaction with a yield of 87.1%.
Synthesis Reference(s)
Canadian Journal of Chemistry, 31, p. 775, 1953 DOI: 10.1139/v53-106
The Journal of Organic Chemistry, 61, p. 1493, 1996 DOI: 10.1021/jo951219c
General Description
Indole-3-carbinol is a novel secondary plant metabolite produced in cruciferous vegetables, such as cabbage, cauliflower and brussels sprouts.
Biochem/physiol Actions
Inhibits cancinogenesis at the initiation stage. Has be shown to inhibit carcinogenesis in several animal species, but it enhances tumor incidence if administered at a post-initiation stage. Found in cruciferous vegetables.
Anticancer Research
I3C is a bioactive compound majorly found in Brassica vegetables including broccoli,cauliflower, and collard greens. I3C and its derivative diindolylmethane (DIM)have been investigated for cancer prevention and treatment of breast, prostate, andovarian cancers. I3C partially modulates the tyrosine kinase/PI3K/Akt signalingpathway which leads to the prevention of lung adenocarcinoma which is induced byusing tobacco carcinogen in A/J mice. DIM transduces signaling via aryl hydrocarbon(Ah) receptor, NF-κB/Wnt/Akt/mTOR signaling pathways, cell cycle arrest,modulated cytochrome P450 enzymes, and altered angiogenetic and invasive,metastatic, and epigenetic behavior of cancer cells. Combination of DIM and I3Cinduces Nrf2-mediated phase II drug metabolizing genes (GSTm2, UGT1A1, andNQO1) and antioxidant genes (HO-1, SOD-1) (Weng et al. 2008; 2012).
Properties of Indole-3-carbinol
| Melting point: | 96-99 °C (lit.) |
| Boiling point: | 267.28°C (rough estimate) |
| Density | 1.1135 (rough estimate) |
| refractive index | 1.5670 (estimate) |
| storage temp. | 2-8°C |
| solubility | soluble in Methanol |
| form | Crystalline Powder or Flakes |
| pka | 15.10±0.10(Predicted) |
| color | Off-white to yellow-orange |
| BRN | 121323 |
| Stability: | 2-80C |
| InChI | InChI=1S/C9H9NO/c11-6-7-5-10-9-4-2-1-3-8(7)9/h1-5,10-11H,6H2 |
| CAS DataBase Reference | 700-06-1(CAS DataBase Reference) |
Safety information for Indole-3-carbinol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Indole-3-carbinol
| InChIKey | IVYPNXXAYMYVSP-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=CC=C2)C(CO)=C1 |
Indole-3-carbinol manufacturer
CEFA CILINAS BIOTICS PVT LTD
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 700-06-1 Indole-3-Carbinol 98%View Details
700-06-1 Indole-3-Carbinol 98%View Details
700-06-1 -
 1H-Indole-3-methanol 99%View Details
1H-Indole-3-methanol 99%View Details
700-06-1 -
 3-Indolemethanol CAS 700-06-1View Details
3-Indolemethanol CAS 700-06-1View Details
700-06-1 -
 700-06-1 98%View Details
700-06-1 98%View Details
700-06-1 -
 700-06-1 99.00%View Details
700-06-1 99.00%View Details
700-06-1 -
 1H-Indole-3-methanol 700-06-1 98%View Details
1H-Indole-3-methanol 700-06-1 98%View Details
700-06-1 -
 Indole-3-carbinol, 96% CAS 700-06-1View Details
Indole-3-carbinol, 96% CAS 700-06-1View Details
700-06-1 -
 Indole-3-carbinol CAS 700-06-1View Details
Indole-3-carbinol CAS 700-06-1View Details
700-06-1
