Indole-5-carboxylic acid
Synonym(s):5-Carboxyindole
- CAS NO.:1670-81-1
- Empirical Formula: C9H7NO2
- Molecular Weight: 161.16
- MDL number: MFCD00005678
- EINECS: 216-799-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:13:45
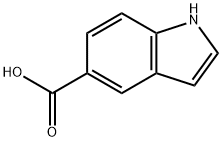
What is Indole-5-carboxylic acid?
Chemical properties
light beige to yellow powder
The Uses of Indole-5-carboxylic acid
Indole-5-carboxylic acid is the suitable reagent used to study the intermolecular excited state proton transfer in indole-2-carboxylic acid and indole-5-carboxylic acid in various solvents in acidic, basic, and neutral media by steady state and time resolved fluorescence spectroscopy. It may be used in the electrochemical synthesis of poly(indole-5-carboxylic acid) (PICA) films. Also used as reactant for preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles, as potential anticancer immunomodulators, synthesis of indirubin derivatives and amide conjugates with ketoprofen, as inhibitors of Gli1-mediated transcription in Hedgehog pathway.
The Uses of Indole-5-carboxylic acid
Reactant for preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles, as potential anticancer immunomodulators 1 Reactant for preparation of indolyl-quinolines via metal- and solvent-free autoxidative coupling reaction 2 Reactant for preparation of anthranilic acids using bromamine-B oxidant and palladium chloride catalyst 3 Reactant for synthesis of indirubin derivatives 4 Reactant for preparation of amide conjugates with ketoprofen, as inhibitors of Gli1-mediated transcription in Hedgehog pathway 5 Reactant for preparation of piperazine-bisamide analogs as human growth hormone secretagogue receptor antagonists for treatment of obesity.
The Uses of Indole-5-carboxylic acid
Indole-5-carboxylic acid is the suitable reagent used to study the intramolecular excited state proton transfer in indole-2-carboxylic acid and indole-5-carboxylic acid in various solvents in acidic, basic, and neutral media by steady state and time resolved fluorescence spectroscopy. It may be used in the electrochemical synthesis of poly(indole-5-carboxylic acid) (PICA) films.
Definition
ChEBI: Indole-5-carboxylic acid is an indolecarboxylic acid in which the carboxy group is the only substituent and is located at position 5. It has a role as a plant metabolite.
General Description
Indole-5-carboxylic acid is an indole derivative. On electropolymerization, it affords electroactive polymer film of poly(indole-5-carboxylic-acid). Different concentrations of indole-5-carboxylic acid in sulfuric acid solution has been investigated for the preventive action against mild steel corrosion. On electropolymerization it affords a trimeric product. Characterization studies of the trimeric product by 1H NMR and various one- and two-dimensional NMR techniques have been reported.
Properties of Indole-5-carboxylic acid
| Melting point: | 211-213 °C (lit.) |
| Boiling point: | 287.44°C (rough estimate) |
| Density | 1.2480 (rough estimate) |
| refractive index | 1.5050 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Soluble in ethanol (50 mg/ml), dimethyl sulfoxide and methanol. |
| pka | 4.40±0.30(Predicted) |
| form | Powder |
| color | Light beige to yellow |
| BRN | 124391 |
| CAS DataBase Reference | 1670-81-1(CAS DataBase Reference) |
Safety information for Indole-5-carboxylic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Indole-5-carboxylic acid
| InChIKey | IENZCGNHSIMFJE-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 1670-81-1 Indole-5-carboxylic acid 98%View Details
1670-81-1 Indole-5-carboxylic acid 98%View Details
1670-81-1 -
 1670-81-1 99%View Details
1670-81-1 99%View Details
1670-81-1 -
 1H-Indole-5-carboxylic acid 1670-81-1 98%View Details
1H-Indole-5-carboxylic acid 1670-81-1 98%View Details
1670-81-1 -
 Indole-5-carboxylic acid 98% CAS 1670-81-1View Details
Indole-5-carboxylic acid 98% CAS 1670-81-1View Details
1670-81-1 -
 Indole-5-carboxylic Acid CAS 1670-81-1View Details
Indole-5-carboxylic Acid CAS 1670-81-1View Details
1670-81-1 -
 Indole-5-carboxylic acid >98% (HPLC) CAS 1670-81-1View Details
Indole-5-carboxylic acid >98% (HPLC) CAS 1670-81-1View Details
1670-81-1 -
 Indole-5-carboxylic acid, 95% CAS 1670-81-1View Details
Indole-5-carboxylic acid, 95% CAS 1670-81-1View Details
1670-81-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
