Indolo[3,2-b]carbazole
- CAS NO.:6336-32-9
- Empirical Formula: C18H12N2
- Molecular Weight: 256.3
- MDL number: MFCD09879258
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:13:47
![Indolo[3,2-b]carbazole Structural](https://img.chemicalbook.in/CAS/GIF/6336-32-9.gif)
What is Indolo[3,2-b]carbazole?
Description
indolo[3,2-b]carbazole has a large planar and rigid conjugated structure with fused alternating three benzene and two pyrrole rings. It is a heterocyclic analogue of pentacene – one of the most used organic semiconductors – and is considered to be a nitrogen-containing electron-rich heteroacene with a ladder-type structure. It is a potent agonist of the aryl hydrocarbon receptor (AhR) and was found in Brassica family vegetables. Since its electron-donating nature, indolo[3,2-b]carbazole derivative is commonly used as hole transporting, charge injection, host, and electroluminescent materials for OLED and OFET devices with good thermal, chemical, and environmental stability[1].
The Uses of Indolo[3,2-b]carbazole
Indolo[3,2-b]carbazole (ICZ) is a popular class of organic materials for various electronic applications. It has been used in a number of devices such as organic light-emitting diodes (OLEDs), organic field-effect transistors (OFETs), solar cells, etc. ICZ is an important unit to synthesize various oligomers and polymers with interesting electrical, optical, magnetic and other features[2].
What are the applications of Application
Indolo[3,2-b]carbazole is a useful biochemical for proteomics research
Benefits
The nitrogen heteroatoms open up the possibility of functionalization and sensing capabilities, as well as lowering the HOMO energy levels, which renders them air-stable. Additionally, Indolo[3,2-b]carbazole exhibit very strong Nsingle bondH?π interactions, which can enhance charge transport by improving intermolecular interactions and thus result in an improved molecular packing motive. Because of the high glass transition temperature, ICZ compounds possess great thermal and morphological stability under ambient conditions[1].
References
[1] Streckaite S, et al. Fluorescence quenching of indolo[3,2-b]carbazole compounds by conformational motions of attached substituents. Dyes and Pigments, 2016; 133: 120-126.
[2] Bintinger J, et al. Synthesis, characterization and printing application of alkylated indolo[3,2-b]carbazoles. Synthetic Metals, 2017; 288: 9-17.
Properties of Indolo[3,2-b]carbazole
| Melting point: | 460°C(lit.) |
| Boiling point: | 569.8±23.0 °C(Predicted) |
| Density | 1.404±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| form | powder to crystal |
| pka | 16.70±0.30(Predicted) |
| color | White to Yellow to Green |
| λmax | 338nm(DMF)(lit.) |
| InChI | InChI=1S/C18H12N2/c1-3-7-15-11(5-1)13-9-18-14(10-17(13)19-15)12-6-2-4-8-16(12)20-18/h1-10,19-20H |
Safety information for Indolo[3,2-b]carbazole
Computed Descriptors for Indolo[3,2-b]carbazole
| InChIKey | YCPBCVTUBBBNJJ-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=CC=C2)C2=C1C=C1C3=C(NC1=C2)C=CC=C3 |
Indolo[3,2-b]carbazole manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![11,12-Dihydro-11-phenylindolo[2,3-a]carbazole](https://img.chemicalbook.in/CAS/GIF/1024598-06-8.gif)
![5,7-Dihydro-7,7-dimethyl-indeno[2,1-b]carbazole](https://img.chemicalbook.in/CAS/GIF/1257220-47-5.gif)
![Indeno[1,2-b]carbazole, 5,11-dihydro-11,11-diMethyl-](https://img.chemicalbook.in/CAS/GIF/1260228-95-2.gif)
![Indolo[3,2-b]carbazole, 5,11-dihydro-5,11-diphenyl-](https://img.chemicalbook.in/CAS/20180702/GIF/58328-30-6.gif)
![5-phenyl-5,12- dihydroindolo [3,2-a]carbazole](https://img.chemicalbook.in/CAS/20180808/GIF/1247053-55-9.gif)
![11,12-DIHYRDOINDOLO[2,3-A]CARBAZOLE](https://img.chemicalbook.in/CAS/GIF/60511-85-5.gif)
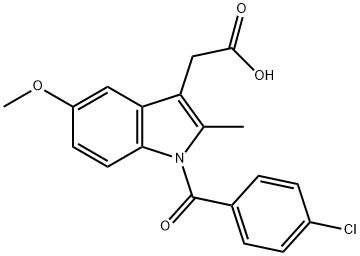
![Indolo[2,3-a]quinolizine,1-ethyl-2,3,4,6,7,12-hexahydro-](https://img.chemicalbook.in/CAS/GIF/40163-47-1.gif)
You may like
-
![5,11-Dihydroindolo[3,2-b]carbazole CAS 6336-32-9](https://img.chemicalbook.in//Content/image/CP5.jpg) 5,11-Dihydroindolo[3,2-b]carbazole CAS 6336-32-9View Details
5,11-Dihydroindolo[3,2-b]carbazole CAS 6336-32-9View Details
6336-32-9 -
 5,11- Dihydroindolo(3,2-b)carbazole (6336-32-9), Purity: 99.30%View Details
5,11- Dihydroindolo(3,2-b)carbazole (6336-32-9), Purity: 99.30%View Details
6336-32-9 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
