1-Hexadecanol
Synonym(s):1-Hexadecanol;Cetyl alcohol;Palmityl alcohol
- CAS NO.:36653-82-4
- Empirical Formula: C16H34O
- Molecular Weight: 242.44
- MDL number: MFCD00004760
- EINECS: 253-149-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:47
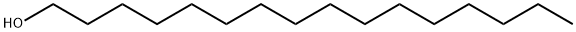
What is 1-Hexadecanol?
Absorption
Following ingestion at a dose level of 2.0 g/kg in rats, cetyl alcohol was partly absorbed . Administration of 0.2 mg cetyl alcohol in rat by stomach tube indicated good absorption as 63-96 % of radiolabeled cetyl alcohol was detected in the lymph . About 15% of total cetyl alcohol was unchanged during its passage through the mucosal cells of the small intestine but mostly underwent oxidation to palmitic acid . The extent of absorption was reported to be 26% in poultry .
Toxicity
Acute oral LD50 in mouse is 3200 mg/kg and acute dermal LD50 is >2600 mg/kg in rabbit . It is considered to be slightly toxic in humans in case of oral ingestion at doses of 5 g/kg and greater . In a rat study, the effects of toxicity were mainly in the central nervous system (CNS) leading to CNS depression and difficulty in respiration after 7-14 days post-administration via stomach tube . In a subchronic dermal toxicity study of rabbits, topical application of 11.5 % cetyl alcohol was associated with histological findings of keratosis, hyperkeratosis, and papillary projections of the epidermis, all of which are features of exfoliative dermatitis . However it is concluded that there is no evidence of major skin irritation and systemic toxicity of cetyl alcohol. Inhalation of 26 ppm cetyl alcohol vapors in animals caused slight irritation of the mucous membranes of the eyes, nose, throat, and respiratory passages . Cetyl Alcohol was not mutagenic in Salmonella typhimurium LT2 mutant strains in the spot test .
Description
1-Hexadecanol is a waxy white powder or flake form at room temperature, and is insoluble in water and soluble in alcohols and oils. Discovered by Chevrenl in 1913, It is one of the oldest known long-chain alcohol. It can be produced from the reduction of palmitic acid. 1-Hexadecanol may be contained in cosmetic and personal care products such as shampoos, creams and lotions. it is mainly used as an opacifier, emulsifier, and thickening agent that alter the thickness of the liquid, and increase and stabilize the foaming capacity.
Chemical properties
Cetyl alcohol occurs as waxy, white flakes, granules, cubes, or castings. It has a faint characteristic odor and bland taste.
Originator
Hexadecyl alcohol,Esso Res. And Eng. Co.
Occurrence
Reported as a major constituent of spermaceti oil, where it is present chiefy as cetyl palmitate Also reported found in guava, peach, pear, kohlrabi, baked potato, mustard, Parmesan cheese, butter, milk powder, boiled egg, cooked chicken, roasted beef, beef fat, whiskies, tea, starfruit, mango, rice, licorice, kiwifruit, loquat, endive, shrimp, crab, clam, Cape gooseberry and pawpaw
The Uses of 1-Hexadecanol
1-Hexadecanol has been used in preparation of:
(±)-2-methoxyheptadecanoic acid (fatty acid)
high-chain fatty acid esters of 1-hexadecanol, novel organic phase change material for thermal energy storage
hexadecane (alkane) in the presence of membrane fraction of Vibrio furnissii M1
The Uses of 1-Hexadecanol
cetyl alcohol is a versatile ingredient that can serve as an emollient, emulsifier, thickener, binder, foam booster, or emulsion stabilizer, depending on the formulation and need. It is derived from coconut or palm oil as well as being synthetically manufactured. It is considered by some sources to be a non-comedogenic material.
Indications
No therapeutic indications in medicinal products. Indicated to be used as an indirect additive in food contact substances, or an ingredient in commercial or cosmetic products.
Background
Cetyl alcohol, also known as 1-hexadecanol or n-hexadecyl alcohol, is a 16-C fatty alcohol with the chemical formula CH3(CH2)15OH. It can be produced from the reduction of palmitic acid. Cetyl alcohol is present in a waxy white powder or flake form at room temperature, and is insoluble in water and soluble in alcohols and oils . Discovered by Chevrenl in 1913, cetyl alcohol is one of the oldest known long-chain alcohol . It may be contained in cosmetic and personal care products such as shampoos, creams and lotions. Mainly it is used as an opacifier, emulsifier, and thickening agent that alter the thickness of the liquid, and increase and stabilize the foaming capacity. Due to its water-binding property, cetyl alcohol is commonly used as an emollient that prevents drying and chapping of the skin . According to the FDA Code of Federal Regulations, cetyl alcohol is a safe synthetic fatty acid in food and in the synthesis of food components under the condition that it contain not less than 98 percent of total alcohols and not less than 94 percent of straight chain alcohols . Cetyl alcohol is also listed in the OTC ingredient list as a skin protectant for skin irritations caused by poison ivy, oak, sumac, and insect bites or stings . Cetyl alcohol is reported to be a mild skin or eye irritant.
Definition
ChEBI: 1-Hexadecanol is a long chain fatty alcohol that is hexadecane substituted by a hydroxy group at position 1. It is a synthetic, solid, fatty alcohol and nonionic surfactant. It is used as an emulsifying agent in pharmaceutical preparations.
Production Methods
Cetyl alcohol may be manufactured by a number of methods such as esterification and hydrogenolysis of fatty acids or by catalytic hydrogenation of the triglycerides obtained from coconut oil or tallow. Cetyl alcohol may be purified by crystallization and distillation.
Therapeutic Function
Pharmaceutic aid
Synthesis Reference(s)
The Journal of Organic Chemistry, 42, p. 512, 1977 DOI: 10.1021/jo00423a025
Synthetic Communications, 25, p. 1901, 1995 DOI: 10.1080/00397919508015865
Tetrahedron Letters, 24, p. 4485, 1983 DOI: 10.1016/S0040-4039(00)85933-X
General Description
1-Hexadecanol is a free fatty acid alcohol generally used as an emulsifier, emollient, opacifier and surfactant in cosmetics formulations.
Flammability and Explosibility
Not classified
Pharmacokinetics
Cetyl alcohol exhibits skin protect properties against skin irritations caused by bites, rashes and stings. The inhibitory action of cetyl alcohol against the growth of Mycoplasma gallisepticum and Mycopiasma pneumoniae has been reported .
Safety
Cetyl alcohol is mainly used in topical formulations, although it has
also been used in oral and rectal preparations.
Cetyl alcohol has been associated with allergic delayed-type
hypersensitivity reactions in patients with stasis dermatitis. Crosssensitization
with cetostearyl alcohol, lanolin, and stearyl alcohol
has also been reported. It has been suggested that hypersensitivity
may be caused by impurities in commercial grades of cetyl
alcohol since highly refined cetyl alcohol (99.5%) has not been
associated with hypersensitivity reactions.
LD50 (mouse, IP): 1.6 g/kg
LD50 (mouse, oral): 3.2 g/kg
LD50 (rat, IP): 1.6 g/kg
LD50 (rat, oral): 5 g/kg
Metabolism
Following ingestion at a dose level of 2.0 g/kg in rats, cetyl alcohol was partly metabolized to palmitic acid . After administration of 0.2 mg cetyl alcohol in rat by stomach tube, cetyl alcohol was mostly oxidized to palmitic acid and incorporated into triglycerides and phospholipids during its passage through the mucosal cells of the small intestine .
Storage
Cetyl alcohol is stable in the presence of acids, alkalis, light, and air; it does not become rancid. It should be stored in a well-closed container in a cool, dry place.
Purification Methods
Crystallise the alcohol from aqueous EtOH or from cyclohexane. Alternatively purify it by zone refining. The purity can be checked by gas chromatography. [Beilstein 1 H 429, 1 I 219, 1 II 466, 1 III 1815, 1 IV 1876.]
Incompatibilities
Incompatible with strong oxidizing agents. Cetyl alcohol is responsible for lowering the melting point of ibuprofen, which results in sticking tendencies during the process of film coating ibuprofen crystals.
Regulatory Status
Included in the FDA Inactive Ingredients Database (ophthalmic preparations, oral capsules and tablets, otic and rectal preparations, topical aerosols, creams, emulsions, ointments and solutions, and vaginal preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
Properties of 1-Hexadecanol
| Melting point: | 48-50 °C (lit.) |
| Boiling point: | 179-181 °C/10 mmHg (lit.) |
| Density | 0.818 g/mL at 25 °C (lit.) |
| vapor density | 8.34 (vs air) |
| vapor pressure | <0.01 mm Hg ( 43 °C) |
| refractive index | nD79 1.4283 |
| FEMA | 2554 | 1-HEXADECANOL |
| Flash point: | 275 °F |
| storage temp. | Store below +30°C. |
| solubility | Soluble in alcohol, chloroform, ether |
| form | Powder, Flakes or Pellets |
| pka | 15.20±0.10(Predicted) |
| color | White to off-white |
| Odor | at 100.00 %. waxy clean greasy floral oily |
| explosive limit | 8% |
| Water Solubility | insoluble |
| JECFA Number | 114 |
| Merck | 14,2028 |
| BRN | 1748475 |
| Dielectric constant | 3.6(60℃) |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong acids. |
| CAS DataBase Reference | 36653-82-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Hexadecanol(36653-82-4) |
| EPA Substance Registry System | 1-Hexadecanol (36653-82-4) |
Safety information for 1-Hexadecanol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for 1-Hexadecanol
1-Hexadecanol manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 CETYL ALCOHOL. 99%View Details
CETYL ALCOHOL. 99%View Details -
 CETYL ALCOHOL 99%View Details
CETYL ALCOHOL 99%View Details -
 Cetyl Alcohol (SQ) CAS 36653-82-4View Details
Cetyl Alcohol (SQ) CAS 36653-82-4View Details
36653-82-4 -
 Cetyl Alcohol pure CAS 36653-82-4View Details
Cetyl Alcohol pure CAS 36653-82-4View Details
36653-82-4 -
 Cetyl Alcohol PowderView Details
Cetyl Alcohol PowderView Details
36653-82-4 -
 Cetyl Alcohol Ginol 16View Details
Cetyl Alcohol Ginol 16View Details
36653-82-4 -
 Cetyl Alcohol, Grade Standard: Industrial GradeView Details
Cetyl Alcohol, Grade Standard: Industrial GradeView Details
36653-82-4 -
 Cetyl Alcohol, 99%, 25 kg bag, for industrial useView Details
Cetyl Alcohol, 99%, 25 kg bag, for industrial useView Details
36653-82-4
