Myristic acid
Synonym(s):1-Tridecanecarboxylic acid;C14:0;Myristic acid;NSC 5028;Tetradecanoic acid
- CAS NO.:544-63-8
- Empirical Formula: C14H28O2
- Molecular Weight: 228.37
- MDL number: MFCD00002744
- EINECS: 208-875-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
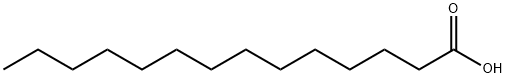
What is Myristic acid?
Description
Myristic acid, also called tetradecanoic acid , is a common saturated fatty acid with the molecular formula CH3(CH2)12COOH. A myristate is a salt or ester of myristic acid.
Myristic acid is named after the nutmeg Myristica fragrans. Nutmeg butter is 75 % trimyristin, the triglyceride of myristic acid. Besides nutmeg, myristic acid is also found in palm kernel oil, coconut oil, butter fat and is a minor component of many other animal fats. It is also found in spermaceti, the crystallized fraction of oil from the sperm whale.
Myristic acid is also commonly added co-translationally to the penultimate, nitrogen-terminus, glycine in receptor-associated kinases to confer the membrane localisation of the enzyme. The myristic acid has a sufficiently high hydrophobicity to become incorporated into the fatty acyl core of the phospholipid bilayer of the plasma membrane of the eukaryotic cell. In this way, myristic acid acts as a lipid anchor in biomembranes.
The ester isopropyl myristate is used in cosmetic and topical medicinal preparations where good absorption through the skin is desired.
Reduction of myristic acid yields myristyl aldehyde and myristyl alcohol.
Description
Lauric acid [1] and myristic acid [2] are saturated fatty acids. Their formal names are dodecanoic acid and tetradecanoic acid, respectively. Both are white solids that are very slightly soluble in water.
Lauric acid esters (principally triglycerides) are found only in vegetable fats, primarily from coconut milk and oil, laurel oil, and palm kernel oil. In contrast, myristic acid triglycerides occur in plants and animals, notably in nutmeg butter, coconut oil, and mammalian milk.
Fatty acids have a bad name because they are strongly associated with high serum cholesterol levels in humans. Lauric and myristic acids are among the worst offenders; therefore, many governmental and health organizations advise that coconut oil and milk, among other high–saturated fat substances, should be excluded from the diet.
There is, however, a bright side for these acids. May 8 is?National Coconut Cream Pie Day, perhaps the one day of the year you may wish to throw caution to the wind and eat your fill of lauric and myristic acids.
Description
Myristic acid is a 14-carbon saturated fatty acid. It is incorporated into myristoyl coenzyme A (myristoyl-CoA) and transferred by N-myristoyltransferase to the N-terminal glycine of certain proteins either during translation to modify protein activity or post-translationally in apoptotic cells.
Chemical properties
Myristic acid has a faint, waxy, oily odor
Chemical properties
Myristic acid occurs as an oily white crystalline solid with a faint odor.
Chemical properties
Prepared from the fatty acid mixture of palm seed oil.
Occurrence
Reported found in nutmeg, palm seed, sperm whale oil, blue cheese, burley tobacco, cooked beef and chicken, fish, rum, apricot, banana, lemon and grapefruit juice, cranberry, guava, grapes, melon, papaya, raspberry, strawberry fruit and jam, cucumber, tomato, many cheeses, thyme, breads, butter, milk, lamb liver, pork, hop oil, beer, cognac, whiskies, peanut oil, cocoa, tea, coconut meat and milk, cloudberry, beans, passion fruit, mushroom, mango, starfruit, tamarind, kelp, cardamom, rice, buckwheat, watercress, malt, wort, loquat, Bourbon vanilla, lemon balm, shrimp, nectarine, crab, scallop, squid, cape gooseberry, Chinese quince, pawpaw and sweet grass oi
The Uses of Myristic acid
Myristic Acid is a common saturated fatty acid found in nutmeg, palm kernel oil, coconut oil and butter fat.
The Uses of Myristic acid
Myristic acid is a 14-carbon saturated (14:0) fatty acid. In vivo, it is commonly added covalently to the N-terminus of proteins in a co-translational process termed N-myristoylation. In addition, there are examples where N-myristoylation occurs post-translationally, when a hidden myristoylation pattern is exposed.
The Uses of Myristic acid
myristic acid is a surfactant and cleansing agent. When combined with potassium, myristic acid soap provides very good, abundant lather. This is a solid organic acid naturally occurring in butter acids such as nutmeg, oil of lovage, coconut oil, mace oil, and most animal and vegetable fats. Although some sources cite it as having no irritation potential, they do indicate comedogenicity potential.
The Uses of Myristic acid
Myristic Acid is a fatty acid obtained from coconut oil and other fats. it has poor water solubility but is soluble in alcohol, chloro- form, and ether. it is used as a lubricant, binder, and defoaming agent.
What are the applications of Application
Myristic Acid is a 14 carbon saturated fatty acid
Preparation
From fatty acid mixture of palm seed oil
Definition
ChEBI: A straight-chain, fourteen-carbon, long-chain saturated fatty acid mostly found in milk fat.
Production Methods
Myristic acid occurs naturally in nutmeg butter and in most animal and vegetables fats. Synthetically, it may be prepared by electrolysis of methyl hydrogen adipate and decanoic acid or by Maurer oxidation of myristyl alcohol.
Aroma threshold values
Detection: 10 ppm
General Description
Oily white crystalline solid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Myristic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Myristic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Fire Hazard
Myristic acid is probably combustible.
Pharmaceutical Applications
Myristic acid is used in oral and topical pharmaceutical formulations. Myristic acid has been evaluated as a penetration enhancer in melatonin transdermal patches in rats and bupropion formulations on human cadaver skin.Further studies have assessed the suitability of myristic acid in oxymorphone formulations and clobetasol 17-propionate topical applications.Furthermore, polyvinyl alcohol substituted with myristic acid (as well as other fatty acids) at different substitution degrees has been used for the preparation of biodegradable microspheres containing progesterone or indomethacin.
Biochem/physiol Actions
Myristic acid is commonly added via a covalent linkage to the N-terminal glycine of many eukaryotic and viral proteins, a process called myristoylation. Myristoylation enables proteins to bind to cell membranes and facilitates protein-protein interactions. Myristolyation of proteins affect many cellular functions and thus has implications in health and disease .
Safety Profile
Poison by intravenous route. Mutation data reported. An eye and human skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Myristic acid is used in oral and topical pharmaceutical formulations
and is generally regarded as nontoxic and nonirritant at the
levels employed as an excipient. However, myristic acid is reported
to be an eye and skin irritant at high levels and is poisonous by
intravenous administration. Mutation data have also been
reported.
LD50 (mouse, IV): 0.043 g/kg
LD50 (rat, oral): >10 g/kg
Storage
The bulk material should be stored in a well-closed container in a cool, dry, place.
Purification Methods
Purify the acid via the methyl ester (b 153-154o/10mm, n25 1.4350), as for capric acid. [Trachtman & Miller J Am Chem Soc 84 4828 1962.] Also purify it by zone melting. It crystallises from pet ether, and is dried in a vacuum desiccator containing shredded wax. [Beilstein 2 IV 1126.]
Incompatibilities
Myristic acid is incompatible with strong oxidizing agents and bases.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (oral capsules). Included in nonparenteral medicines licensed in the UK.
Properties of Myristic acid
| Melting point: | 52-54 °C(lit.) |
| Boiling point: | 250 °C100 mm Hg(lit.) |
| Density | 0.862 |
| vapor pressure | <0.01 hPa (20 °C) |
| FEMA | 2764 | MYRISTIC ACID |
| refractive index | nD60 1.4305; nD70 1.4273 |
| Flash point: | >230 °F |
| storage temp. | room temp |
| solubility | 1.07mg/l |
| form | Flakes, Powder, Chunks or Crystalline Mass |
| appearance | white crystals or powder |
| pka | 4.78±0.10(Predicted) |
| color | White |
| Odor | at 100.00 %. waxy fatty soapy coconut |
| Water Solubility | <0.1 g/100 mL at 18 ºC |
| Merck | 14,6333 |
| JECFA Number | 113 |
| BRN | 508624 |
| Stability: | Stable. Incompatible with strong oxidizing agents, bases. |
| CAS DataBase Reference | 544-63-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Tetradecanoic acid(544-63-8) |
| EPA Substance Registry System | Myristic acid (544-63-8) |
Safety information for Myristic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Myristic acid
Myristic acid manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

![(aR)-alpha-[(1S)-1-[2,6-Dimethoxy-4-(2-propen-1-yl)phenoxy]ethyl]-4-hydroxy-3-methoxybenzenemethanol](https://img.chemicalbook.in/CAS/GIF/171485-39-5.gif)
You may like
-
 544-63-8 98%View Details
544-63-8 98%View Details
544-63-8 -
 Myristic Acid CAS 544-63-8View Details
Myristic Acid CAS 544-63-8View Details
544-63-8 -
 Myristic Acid pure CAS 544-63-8View Details
Myristic Acid pure CAS 544-63-8View Details
544-63-8 -
 Myristic Acid (High Purity) extrapure CAS 544-63-8View Details
Myristic Acid (High Purity) extrapure CAS 544-63-8View Details
544-63-8 -
 Myristic Acid CASView Details
Myristic Acid CASView Details -
 Myristic acid CAS 544-63-8View Details
Myristic acid CAS 544-63-8View Details
544-63-8 -
 Myristic Acid SolidView Details
Myristic Acid SolidView Details
544-63-8 -
 Godrej Myristic Acid 99%View Details
Godrej Myristic Acid 99%View Details
544-63-8
