n-Hendecane
Synonym(s):n-Undecane;Hendecane;n-Undecane;Undecane
- CAS NO.:1120-21-4
- Empirical Formula: C11H24
- Molecular Weight: 156.31
- MDL number: MFCD00008959
- EINECS: 214-300-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
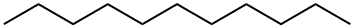
What is n-Hendecane?
Chemical properties
colourless liquid.
The Uses of n-Hendecane
Petroleum research, organic synthesis, distillation chaser.
The Uses of n-Hendecane
Undecane is used in preparation method of small-size hollow Silicon Dioxide.
The Uses of n-Hendecane
Undecane is mainly used as a model n-alkane in studies relating to viscosities, excess molar enthalpies and vapor-liquid equilibrium of binary alkane mixtures.
Production Methods
Undecane is obtained from the refining of petroleum. Paraffins are isolated by selective adsorption followed by fractional distillation to produce the desired mix of nparaffins (63).
Definition
ChEBI: Undecane is a straight-chain alkane with 11 carbon atoms.
Synthesis Reference(s)
Journal of the American Chemical Society, 95, p. 6131, 1973 DOI: 10.1021/ja00799a058
The Journal of Organic Chemistry, 50, p. 3082, 1985
General Description
A colorless liquid. Insoluble in water and less dense than water. Flash point 130°F. Used to make other chemicals.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
Saturated aliphatic hydrocarbons, such as n-Hendecane, may be incompatible with strong oxidizing agents like nitric acid. Charring of the hydrocarbon may occur followed by ignition of unreacted hydrocarbon and other nearby combustibles. In other settings, aliphatic saturated hydrocarbons are mostly unreactive. They are not affected by aqueous solutions of acids, alkalis, most oxidizing agents, and most reducing agents.
Health Hazard
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Safety Profile
Moderately toxic by intravenous route. Flammable liquid when exposed to heat, sparks, flame, or oxidizers.To fight fire, use foam, mist, dry chemical. Emitted from modern buildmg materials (CENEAR 69,22,91). When heated to decomposition it emits acrid smoke and irritating fumes. See also ALKANES.
Carcinogenicity
Undecane (25 mg) and benzo[a] pyrene (B[a]P) (5 mg) were applied to the skin of female ICR/ Ha Swiss mice for 3/week for 440 days, inducing papillomas in 41 of 50 animals. B[a]P alone induced tumors in 12 of 50 animals in the same time, while undecane alone did not produce tumors.
Properties of n-Hendecane
| Melting point: | -26 °C (lit.) |
| Boiling point: | 196 °C (lit.) |
| Density | 0.74 g/mL at 25 °C (lit.) |
| vapor density | 5.4 (vs air) |
| vapor pressure | <0.4 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 140 °F |
| storage temp. | Store below +30°C. |
| form | Liquid |
| color | Colorless |
| Specific Gravity | 0.7402 |
| Odor Threshold | 0.62ppm |
| explosive limit | 0.6-6.5%(V) |
| Water Solubility | IMMISCIBLE |
| BRN | 1697099 |
| Dielectric constant | 2.0(20℃) |
| CAS DataBase Reference | 1120-21-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Undecane(1120-21-4) |
| EPA Substance Registry System | Undecane (1120-21-4) |
Safety information for n-Hendecane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids H304:Aspiration hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. |
Computed Descriptors for n-Hendecane
| InChIKey | RSJKGSCJYJTIGS-UHFFFAOYSA-N |
n-Hendecane manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 n-Undecane CAS 1120-21-4View Details
n-Undecane CAS 1120-21-4View Details
1120-21-4 -
 n-Undecane CAS 1120-21-4View Details
n-Undecane CAS 1120-21-4View Details
1120-21-4 -
 n-Undecane CAS 1120-21-4View Details
n-Undecane CAS 1120-21-4View Details
1120-21-4 -
![Undecane [Standard Material for GC] CAS 1120-21-4](https://img.chemicalbook.in//Content/image/CP5.jpg) Undecane [Standard Material for GC] CAS 1120-21-4View Details
Undecane [Standard Material for GC] CAS 1120-21-4View Details
1120-21-4 -
 Undecane CAS 1120-21-4View Details
Undecane CAS 1120-21-4View Details
1120-21-4 -
 n-Undecane, GR 99% CAS 1120-21-4View Details
n-Undecane, GR 99% CAS 1120-21-4View Details
1120-21-4 -
 Undecane CAS 1120-21-4View Details
Undecane CAS 1120-21-4View Details
1120-21-4 -
 n-Undecane CAS 1120-21-4View Details
n-Undecane CAS 1120-21-4View Details
1120-21-4
