1-Bromonaphthalene
Synonym(s):α-Naphthyl bromide;1-Bromonaphthalene
- CAS NO.:90-11-9
- Empirical Formula: C10H7Br
- Molecular Weight: 207.07
- MDL number: MFCD00003868
- EINECS: 201-965-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-27 17:16:38
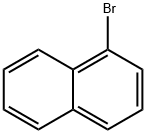
What is 1-Bromonaphthalene?
Chemical properties
Thick colorless yellow to yellow-brown liquid with a pungent odor. Insoluble in water, miscible with alcohol, ether, benzene and chloroform. 1-Bromonaphthalene is one of two isomeric bromonaphthalenes, the other being 2-bromonaphthalene.
The Uses of 1-Bromonaphthalene
1-Bromonaphthalene is used in organic synthesis and refractometry of fats. It is used in the preparation of N-aryl imidazoles and diaryl ethers. It is used to determine the refractive index of crystals and the water content of alcohols. It is utilized in the preparation of glutathione peroxidase-like antioxidant activity of diaryl diselenides. Further, it serves as a solvent for the exfoliation and dispersion of hexabenzocoronene. In addition to this, it is used as a refrigerant and a solvent molecular weight substance.
Preparation
1-Bromonaphthalene is synthesized by the reaction of naphthalene with bromine: C10H8+ Br2→ C10H7Br + HBr.
Reaction: add carbon tetrachloride and naphthalene in the reaction pot, stirring and heating, and slowly add bromine at 45℃. After adding, keep the reaction at 70-80℃ for 3-4h. distillation to recover carbon tetrachloride, wash the reaction product, distill under reduced pressure, wash again, filter, dry and get the finished product.
What are the applications of Application
1-Bromonaphthalene is a bromoarene that can be used in:
Palladium-catalyzed Suzuki–Miyaura coupling reaction with potassium aryltrifluoroborates without the use of phase-transfer catalysts or phosphine ligands.
The preparation of indeno annelated polycyclic aromatic hydrocarbons by reacting with o-bromobenzeneboronic acid and oligocyclic bromoarenes via Suzuki-Heck type coupling.
Ni catalyzed Kumada–Tamao–Corriu cross-coupling reaction with PhMgBr.
The preparation of arylnaphthalenes via palladium-catalyzed Suzuki-Miyaura cross-coupling reaction with aryl boronic acid.
Synthesis Reference(s)
Organic Syntheses, Coll. Vol. 1, p. 121, 1941
Tetrahedron, 64, p. 4999, 2008 DOI: 10.1016/j.tet.2008.03.085
General Description
The vibrational spectra of 1-bromonaphthalene has been studied.
Purification Methods
Purify 1-bromonaphthalene by passage through activated alumina, and three vacuum distillations. [Beilstein 5 H 547, 5 IV 1665.]
Properties of 1-Bromonaphthalene
| Melting point: | −2-−1 °C(lit.) |
| Boiling point: | 133-134 °C10 mm Hg(lit.) |
| Density | 1.48 g/mL at 20 °C(lit.) |
| vapor pressure | 0.013 hPa (20 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | H2O: slightly soluble |
| form | Liquid |
| color | slightly yellow to deep brownish-yellow |
| Water Solubility | slightly soluble |
| Merck | 14,1425 |
| BRN | 1906414 |
| Dielectric constant | 5.1(19℃) |
| CAS DataBase Reference | 90-11-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Naphthalene, 1-bromo-(90-11-9) |
| EPA Substance Registry System | Naphthalene, 1-bromo- (90-11-9) |
Safety information for 1-Bromonaphthalene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1-Bromonaphthalene
| InChIKey | DLKQHBOKULLWDQ-UHFFFAOYSA-N |
1-Bromonaphthalene manufacturer
CEFA CILINAS BIOTICS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


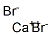


You may like
-
 90-11-9 1-Bromonaphthalene, 97% 98%View Details
90-11-9 1-Bromonaphthalene, 97% 98%View Details
90-11-9 -
 A-BROMONAPHTHALENE 33186 99%View Details
A-BROMONAPHTHALENE 33186 99%View Details
33186 -
 1-Bromonaphthalene pure CAS 90-11-9View Details
1-Bromonaphthalene pure CAS 90-11-9View Details
90-11-9 -
 1-Bromonaphthalene Chemical, LiquidView Details
1-Bromonaphthalene Chemical, LiquidView Details
90-11-9 -
 1-Bromo Naphthalene, Packaging Type: DrumView Details
1-Bromo Naphthalene, Packaging Type: DrumView Details
90-11-9 -
 Sulab Pale Yellow 1-Bromonaphthalene, Packaging: 500ml To 5ltrView Details
Sulab Pale Yellow 1-Bromonaphthalene, Packaging: 500ml To 5ltrView Details
90-11-9 -
 1-Bromonaphthalene CAS: 90-11-9View Details
1-Bromonaphthalene CAS: 90-11-9View Details
90-11-9 -
 1 Bromo Naphthalene, LiquidView Details
1 Bromo Naphthalene, LiquidView Details
90-11-9
