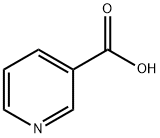
PYRIDINE-2-CARBOXAMIDE
Synonym(s):2-Pyridinecarboxamide;Picolinamide
- CAS NO.:1452-77-3
- Empirical Formula: C6H6N2O
- Molecular Weight: 122.12
- MDL number: MFCD00023483
- EINECS: 215-921-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:59
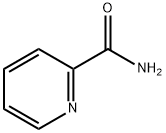
What is PYRIDINE-2-CARBOXAMIDE?
Chemical properties
White to off-white solid
The Uses of PYRIDINE-2-CARBOXAMIDE
A nicotinic acid derivative for prevention and treatment of cancer by activating RUNX3 gene.
The Uses of PYRIDINE-2-CARBOXAMIDE
Picolinamide was used as template in preparation of molecular imprinting polymer. Picolinamide was used in a study to evaluate kinetics and mechanism of liberation of picolinamide from chromium(III)-picolinamide complexes in HClO4.
What are the applications of Application
Picolinamide is a bidentate ligand
Definition
ChEBI: A pyridinecarboxamide that is the monocarboxylic acid amide derivative of picolinic acid.
Biological Activity
picolinamide is a poly (adp-ribose) synthetase (parp) inhibitor.parp inhibitors, a group of pharmacological inhibitors of the enzyme poly adp ribose polymerase (parp), are developed for multiple indications, especially for the treatment of cancer.
Biochem/physiol Actions
Picolinamide is potential inhibitor of poly (ADP-ribose) synthetase of nuclei from rat pancreatic islet cells. Picolinamide acts as bidentate ligand and forms complexes with lanthanide nitrates, thiocyanates and perchlorates.
in vitro
the pathway of oxidation of picolinamide by a gram-negative rod has been elucidated. results showed that under high ph conditions, whole cells could release 2,5-dihydroxypyridine into culture supernatants. moreover, sodium arsenite was able to cause whole cells to accumulate 6-hydroxypicolinate in the culture media. in addition, whole cells were found to oxidize picolinamide, without lag. it was also found that cell-free extracts could convert picolinamide into picolinate, and hydroxylate picolinate into 6-hydroxypicolinate [1].
in vivo
picolinamide was used in a previous study to evaluate the possibility that the inhibition of na+/phosphate cotransport might be associated with the inhibition of nad hydrolyzing enzymes. results showed that the overnight treatment of rats with picolinamide, administered as a single injection (4 mmol/kg), could inhibit na+/phosphate cotransport by isolated renal brush border membrane vesicles. similar to nicotinamide, the inhibition caused by picolinamide occurred in thyroparathyroidectomized rats, was specific for na+/phosphate cotransport. unlike nicotinamide, there was only a small 1.5-fold increase in renal cortical nad content after picolinamide treatment [2].
References
[1] c. g. orpin,m. knight, and w. c. evans. the bacterial oxidation of picolinamide, a photolytic product of diquatbiochem j. 1972 may; 127(5): 819–831.
[2] campbell pi, al-mahrouq ha,abraham mi,kempson sa. specific inhibition of rat renal na+/phosphate cotransport by picolinamide. j pharmacol exp ther.1989 oct;251(1):188-92.
Properties of PYRIDINE-2-CARBOXAMIDE
| Melting point: | 110 °C (dec.)(lit.) |
| Boiling point: | 143 °C / 20mmHg |
| Density | 1.2236 (rough estimate) |
| refractive index | 1.5350 (estimate) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | pK1: 2.10(+1) (20°C) |
| form | Solid |
| color | White to Off-White |
| BRN | 109617 |
| CAS DataBase Reference | 1452-77-3(CAS DataBase Reference) |
| EPA Substance Registry System | 2-Pyridinecarboxamide (1452-77-3) |
Safety information for PYRIDINE-2-CARBOXAMIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for PYRIDINE-2-CARBOXAMIDE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Pyridine-2-carboxamide 98% CAS 1452-77-3View Details
Pyridine-2-carboxamide 98% CAS 1452-77-3View Details
1452-77-3 -
 2-Picolinamide CAS 1452-77-3View Details
2-Picolinamide CAS 1452-77-3View Details
1452-77-3 -
 Picolinamide CAS 1452-77-3View Details
Picolinamide CAS 1452-77-3View Details
1452-77-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
