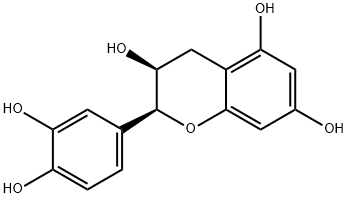
(-)-Epicatechin gallate
Synonym(s):(−)-cis-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol 3-gallate;(−)-cis-3,3′,4′,5,7-Pentahydroxyflavane 3-gallate;(−)-Epicatechin gallate;3-O-Galloylepicatechin
- CAS NO.:1257-08-5
- Empirical Formula: C22H18O10
- Molecular Weight: 442.37
- MDL number: MFCD00075936
- EINECS: 603-088-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-22 20:27:16
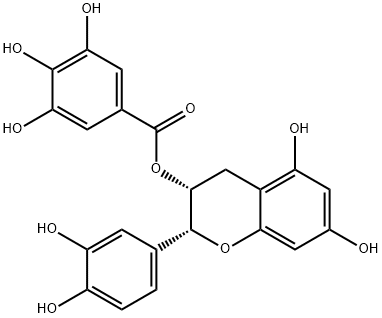
What is (-)-Epicatechin gallate?
Chemical properties
White powder
The Uses of (-)-Epicatechin gallate
(-)-Epicatechin gallate is polyphenols which are abundant in green and black teas. (-)-Epicatechin gallate (ECG) is a natural catechin with a single galloyl group. The hydroxyl groups of ECG contribute to its potent antioxidant activity and facilitate the killing of methicillin-resistant strains of S. aureus.
The Uses of (-)-Epicatechin gallate
One of the catechin isomers and a potent antioxidant that can modulate a wide range of membrane proteins. Its bilayer-modifying potency was tested using gramicidin A (gA) channels as probes. All the c atechins alter gA channel function and modify bilayer properties, with a 500-fold range in potency. The gallate group causes current block, as evident by brief downward current transitions.
The Uses of (-)-Epicatechin gallate
An antioxidant investigated as an adjunct treatment of some methyicillin resistant bacteria.
What are the applications of Application
(?)Epicatechin gallate is an antioxidant investigated as an adjunct treatment of some methyicillin resistant bacteria
Definition
ChEBI: A gallate ester obtained by formal condensation of the carboxy group of gallic acid with the (3R)-hydroxy group of epicatechin. A natural product found in Parapiptadenia rigida.
General Description
(?)-Epicatechin gallate is one of the major polyphenols present in green tea. It belongs to the catechin gallates group of organic compounds. ECG has a chemical structure like that of (?)-epigallocatechin gallate (EGCG), the other major polyphenol found in green tea.
Biological Activity
(-)-epicatechin gallate is a major catechin component in green tea [1].(-)-epicatechin gallate (ecg) plays an important role in cell growth inhibition, apoptosis and membrane transport system [1].(-)-epicatechin gallate is a kind of catechin. in hct-116 cells, ecg activated transcription factor 3 (atf3), which played a critical role in pro-apoptosis. egr-1 was involved in ecg-induced atf3 expression. in hct-116 cells, ecg (50 μm) increased nag-1 and atf3 expression in time- and dose-dependent way [1]. in carcinoma hsc-2 cells, ecg (50 μm) exhibited cytotoxicity with midpoint cytotoxicity (nr50) value of 67 μm. however, in normal hgf-2 fibroblasts, ecg exhibited cytotoxicity at concentrations up to 25 μm with nr50 value of 100 μm. in carcinoma hsc-2 cells, ecg (250 μm) induced nucleosomal dna fragmentation and apoptosis. ecg (150 μm) significantly increased caspase-3 activity [2].in rats, there were five metabolites of ecg: (-)-epicatechin gallate, 3’,4’’-di-o-methyl-(-)-epicatechin gallate, 4’’-o-methyl-(-)-epicatechin gallate, 4’-o-methyl-(-)-epicatechin gallate and 3’-o-methyl-(-)-epicatechin gallate, which were excreted in rat urine [3].
Biochem/physiol Actions
(?)-Epicatechin gallate (ECG), a potent free-radical scavenger has an inhibitory effect on collagenase and elastase enzymes making it an anti-aging agent. It displays antiproliferative effects on human colorectal cells and hence is a chemo-preventative agent. It influences cell survival, preventing apoptosis or autophagy by promoting cell proliferation in oxygen-glucose deprived human brain microvascular endothelial cells. Being structurally and functionally similar to EGCG, ECG might be involved in balancing the mammalian target of rapamycin (mTOR)-AMP-activated protein kinase (AMPK) pathways in endoplasmic reticulum stress. It prevents cell death in this scenario by showing resistance against oxygen-deprived cells. ECG may contribute in preventing oxidative stress. It effectively inhibits secretory sphingomyelinase in many disease states and provides protection for human spermatozoa in Assisted reproductive technology (ART).
References
[1]. cho kn, sukhthankar m, lee sh, et al. green tea catechin (-)-epicatechin gallate induces tumour suppressor protein atf3 via egr-1 activation. eur j cancer, 2007, 43(16): 2404-2412.
[2]. babich h, krupka me, nissim ha, et al. differential in vitro cytotoxicity of (-)-epicatechin gallate (ecg) to cancer and normal cells from the human oral cavity. toxicol in vitro, 2005, 19(2): 231-242.
[3]. kohri t, suzuki m, nanjo f. identification of metabolites of (-)-epicatechin gallate and their metabolic fate in the rat. j agric food chem, 2003, 51(18): 5561-5566.
Properties of (-)-Epicatechin gallate
| Melting point: | 257-258°C |
| Boiling point: | 861.7±65.0 °C(Predicted) |
| alpha | -182~-194°(D/20℃)(c=0.2,CH3OH) |
| Density | 1.80±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Acetone (Sparingly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 7.75±0.25(Predicted) |
| color | White to Off-White |
| Water Solubility | Soluble in water, acetone, DMSO, methanol. |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 1257-08-5(CAS DataBase Reference) |
Safety information for (-)-Epicatechin gallate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for (-)-Epicatechin gallate
| InChIKey | LSHVYAFMTMFKBA-TZIWHRDSSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
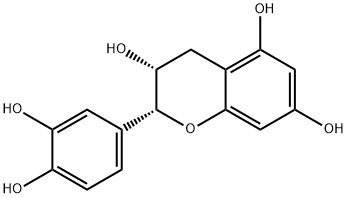
 (-)-Epicatechin Gallate CAS 1257-08-5View Details
(-)-Epicatechin Gallate CAS 1257-08-5View Details
1257-08-5 -
 Epicatechin gallate CAS 1257-08-5View Details
Epicatechin gallate CAS 1257-08-5View Details
1257-08-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1