Pyrogallol
Synonym(s):1,2,3-Trihydroxybenzene;2,3-Dihydroxyphenol;Pyrogallic acid;Pyrogallic acid, 1,2,3-Trihydroxybenzene;Pyrogallol
- CAS NO.:87-66-1
- Empirical Formula: C6H6O3
- Molecular Weight: 126.11
- MDL number: MFCD00002192
- EINECS: 201-762-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
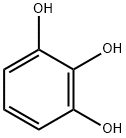
What is Pyrogallol?
Description
Pyrogallol is a natural oxidant that can generate superoxide (O2-) in alkaline solutions through autoxidation to a semiquinone radical. Importantly, the semiquinone radical can react with O2- in an acidic environment to produce a quinone and H2O2. Pyrogallol autoxidation is used in superoxide dismutase activity assays. It can also be used in assays to assess antioxidant capacity. Pyrogallol is used in some biological systems as an O2- scavenger. In other biological systems, it is used as an O2- generator. Pyrogallol effectively scavenges DPPH radical and ABTS+ in vitro. Pyrogallol is a product of tannin degradation to gallic acid by ruminant microbes and has hepatotoxic and nephrotoxic effects in vivo.
Chemical properties
white crystalline solid
Chemical properties
White or nearly white needle- or leaf-shaped crystals or crystalline powder.Pyrogallol is practically odorless.
The Uses of Pyrogallol
Pyrogallol possesses importance as a spectrophotometric reagent in the determination of niobium and tantalum. The absorptions of niobium and tantalum complexes are usually measured at 340 and 335 nm, respectively. The niobium complex is formed in slightly acidic medium, and the tantalum complex in strongly acidic medium (4 N HC1). The absorption spectra are pH-dependen.
The Uses of Pyrogallol
Complexing agent; reducing agent; alkaline solution indicator for gaseous oxygen.
The Uses of Pyrogallol
Pyrogallol is used in the manufacture of various dyes; in dyeing furs, hairs, and feathers; for staining leather; in engraving;as a developer in photography; and as an analytical reagent..
What are the applications of Application
Pyrogallol is a metal-complexing benzene triol compound
Definition
ChEBI: A benzenetriol carrying hydroxy groups at positions 1, 2 and 3.
Production Methods
Pyrogallol is prepared by heating dried gallic acid at about 200°C with the loss of carbon dioxide or by the chlorination of cyclohexanol to tetrachlorocyclohexanone, followed by hydrolysis.
General Description
Odorless white to gray solid. Sinks and mixes with water.
Air & Water Reactions
Turns gray on exposure to light or air. Water soluble.
Reactivity Profile
Pyrogallol is a strong reducing agent. Reacts with alkalis, NH3, antipyrine, camphor, phenol, iron and lead salts, iodine, lime water, menthol and KMnO4.
Hazard
Toxic by ingestion and skin absorption.
Health Hazard
Inhalation of dust causes irritation of nose and throat. Ingestion may cause severe gastrointestinal irritation, convulsions, circulatory collapse, and death. Contact with eyes causes irritation. Skin contact can cause local discoloration, irritation, eczema, and death; repeated contact can cause sensitization.
Health Hazard
The toxic symptoms are similar to those of phenol. It can enter the body by absorption through skin and ingestion. The poisoning effects are nausea, vomiting, gastritis, hemolysis, methemoglobinemia, kidney and liver damage, convulsions, and congestion of lungs. High doses can cause death. Ingestion of 2–3 g of solid can be fatal to humans. The LD50 values varied widely in species. The oral LD50 value in mice is about 300 mg/kg.
Fire Hazard
Pyrogallol is probably combustible.
Contact allergens
Pyrogallol belongs to the phenols group. It is an old photograph developer and a low sensitizer in hair dyes.
Biochem/physiol Actions
Pyrogallol also referred to as 1,2,3-trihydroxybenzene inhibits the response to nitric oxide (NO) in the rat anococcygeus muscle.
Safety Profile
Human poison by ingestion and subcutaneous routes. An experimental poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. 1 198 PPRSOO PYROSULFURYL CHLORIDE Readdy absorbed through the skin. Human systemic effects by ingestion: convulsions, dyspnea, gastrointestinal effects. A severe skin and eye irritant. Incompatible with alkalies, NH3, antipyrine, phenol, iron and lead salts, iodine, KMn04. When heated to decomposition it emits acrid smoke and irritating fumes. Used as a topical antibacterial agent, as an intermediate, hair dye component, and analytical reagent.
Carcinogenicity
Pyrogallol was not carcinogenic
in mouse and rabbit chronic dermal studies. Mice were
treated twice weekly with pyrogallol in acetone (50%) on
the shaved flank for life. There was no increase in dermal or
systemic tumors. A similar study in rabbits also
revealed no skin tumors, although positive controls showed
an increase in tumors in both mice and rabbits.
Pyrogallol was considered to be cocarcinogenic when
administered dermally three times a week together with
the skin carcinogen benzo[a]pyrene for 440 days;
pyrogallol administered alone caused no increase in skin
tumors.
Properties of Pyrogallol
| Melting point: | 43-47 °C(lit.) |
| Boiling point: | 309 °C |
| Density | 1.112 g/mL at 25 °C(lit.) |
| vapor density | 4.4 (vs air) |
| vapor pressure | 10 mm Hg ( 167.7 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | water: soluble |
| form | Very Fine Crystalline Powder |
| pka | pK1:9.03(0);pK2:11.63(+1) (25°C) |
| color | White |
| PH | 4-5 (50g/l, H2O, 20℃) |
| Water Solubility | 400 g/L (25 ºC) |
| Sensitive | Light Sensitive |
| Merck | 14,8000 |
| BRN | 907431 |
| Stability: | Stable, but decolourises in light. Combustible. Incompatible with strong oxidising agents, alkalies, metal oxides, ammonia, antipyrine, phenol, iodine, lime water, menthol, potassium permanganate, strong bases. |
| CAS DataBase Reference | 87-66-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,2,3-Benzenetriol(87-66-1) |
| EPA Substance Registry System | Pyrogallol (87-66-1) |
Safety information for Pyrogallol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H341:Germ cell mutagenicity H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Pyrogallol
| InChIKey | WQGWDDDVZFFDIG-UHFFFAOYSA-N |
Pyrogallol manufacturer
Innovative
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Pyrogallol CAS 87-66-1View Details
Pyrogallol CAS 87-66-1View Details
87-66-1 -
 Pyrogallol CASView Details
Pyrogallol CASView Details -
 Pyrogallol CASView Details
Pyrogallol CASView Details -
 Pyrogallol extrapure AR CAS 87-66-1View Details
Pyrogallol extrapure AR CAS 87-66-1View Details
87-66-1 -
 PyrogallolView Details
PyrogallolView Details
87-66-1 -
 PYROGALLOL LR (1 KG)View Details
PYROGALLOL LR (1 KG)View Details
87-66-1 -
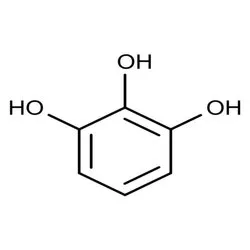 Pyrogallol (CAS Number: 87-66-1), Packaging Size: 1 kg / 5 kg / 10 kg / 25 kgView Details
Pyrogallol (CAS Number: 87-66-1), Packaging Size: 1 kg / 5 kg / 10 kg / 25 kgView Details
87-66-1 -
 Pyrogallol Ar 99%View Details
Pyrogallol Ar 99%View Details
87-66-1
