ZINC PHOSPHIDE
- CAS NO.:1314-84-7
- Empirical Formula: P2Zn3
- Molecular Weight: 258.12
- MDL number: MFCD00049631
- EINECS: 215-244-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-17 17:11:58
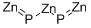
What is ZINC PHOSPHIDE?
Description
Zinc phosphide, trizinc diphosphide, is an amorphous gray-black powder with a garlic-like odor. It is practically insoluble in water (decomposes slowly), ethanol, slightly soluble in carbon disulfide, and benzene [9, p. 967]. Zinc phosphide is produced by heating finely powdered zinc with phosphorus (10).
Description
Zinc phosphide (Zn3P2) is a synthetic inorganic compound that has widely divergent uses. It was originally prepared in the early 20th century by heating stoichiometric amounts of the elements. Much more recently (2013), Erik J. Luber*, Md Hosnay Mobarok, and Jillian M. Buriak* at Canada’s National Institute for Nanotechnology and the University of Alberta (both in Edmonton) prepared pure nanocrystalline Zn3P2 by treating tri-n-octylphosphine (TOP) with dimethylzinc at 320 °C.
Under ambient conditions, zinc phosphide has a tetragonal crystal structure (shown); it converts to cubic when heated to ≈845 °C. This brings up an unusual situation about Zn3P2’s melting point: Some references report that it melts at 1160 °C or higher, but others give 420 °C. The phase-change temperature clearly indicates that the higher melting point is correct.
The original use for zinc phosphide was as a rodenticide, for burrowing pests such as gophers and moles and for domestic pests such as rats and mice. It is usually combined with baits that the animals ingest. Zn3P2 hydrolyzes slowly in contact with water; but the acidity in the target pest’s stomach rapidly releases highly toxic phosphine gas, a respiratory poison. Phosphine was the Molecule of the Week for October 22, 2018.
The newer use of zinc phosphide is more pleasant and exciting. In 2009, Gregory M. Kimball and co-workers at Caltech (Pasadena, CA) reported that steady-state photoluminescence spectra of Zn3P2 wafers have a fundamental indirect band gap of 1.38 eV, close to the ideal direct band gap value of 1.5 eV. This result indicates that it may be valuable as a semiconductor in photovoltaic cells.
The work of Luber et al., mentioned above, also involved photovoltaics. Their synthetic method produced colloidal semiconducting ≈8-nm nanocrystals with the tetragonal (α-Zn3P2) structure. They found that the optical band gap in this form is 0.5 eV greater than that of bulk Zn3P2. Films prepared via deposition of the nanoparticles were used in heterojunction devices that had excellent rectification behavior. Other data, however, indicated that the particles had phosphorus-rich shells (due to the presence of elemental phosphorus [P(0)]), which hindered performance.
Subsequently, Luber, Buriak, and colleagues developed an alternative method for preparing nanocrystalline zinc phosphide that uses tris(trimethylsilyl)phosphine instead of TOP as the phosphorus source. This change greatly decreased the concentration of P(0) on the particle surfaces.
Chemical properties
Dark gray, gritty powder. Stable if dry. Insoluble in alcohol; soluble in acids; decomposes in water.
Chemical properties
Zinc phosphide is a gray crystalline solid.
The Uses of ZINC PHOSPHIDE
Zinc phosphide has been used since the second world war, and is most commonly used as an effective insecticide and rodenticide. Zinc phosphide is highly toxic due to its production of phosphine gas, and can cause symptoms such as nausea, vomiting and dyspnea in those (animal or human) that are exposed.
General Description
ZINC PHOSPHIDE is a dark gray granular solid. ZINC PHOSPHIDE is slowly decomposed by water giving off phosphine, a flammable poison gas. ZINC PHOSPHIDE is toxic by ingestion. ZINC PHOSPHIDE is used in medicine and as a rat poison.
Reactivity Profile
ZINC PHOSPHIDE is a reducing agent. They slowly generate flammable or noxious gases in contact with water. Phosphides react quickly upon contact with moisture or acids to give the very toxic gas phosphine; phosphides also can react vigorously with oxidizing materials. In general, materials in this group are incompatible with oxidizers such as atmospheric oxygen. They are violently incompatible with acids, particularly oxidizing acids.
Health Hazard
ZINC PHOSPHIDE is very caustic when ingested. ZINC PHOSPHIDE reacts with water and acid in the stomach and causes severe irritation. The probable oral lethal dose is 5-50 mg/kg, or between 7 drops and 1 teaspoonful for a 70 kg (150 lb.) person. Most patients die after about 30 hours from peripheral vascular collapse secondary to the compound's direct effects. Extensive liver damage and kidney damage can also occur. Ingestion of 4-5 grams has produced death in human adults, but also doses of 25 to 50 grams have been survived. The lowest oral lethal dose reported for women is 80 mg/kg.
Fire Hazard
When heated to decomposition, ZINC PHOSPHIDE emits toxic fumes of phosphorus and zinc oxides. Irritating oxides of phosphorus may be formed in fires. May ignite in presence of moisture. Contact with water produces flammable gas. Runoff to sewer may create fire or explosion hazard. Decomposed slowly by water giving off phosphine, a flammable poison gas. Reacts violently with concentrated sulfuric acid, nitric acid, and other oxidizing agents. Reacts with hydrochloric acid or sulfuric acid with the evolution of spontaneously flammable phosphine. May ignite in the presence of moisture, or evolve flammable gas. Stable unless exposed to moisture; toxic phosphine gas may then be released and collected in closed spaces. Hazardous polymerization may not occur.
Agricultural Uses
Rodenticide: A U.S. EPA restricted Use Pesticide (RUP). Registered for use in EU countries . Zinc phosphide reacts with the acidic conditions in the gut to form phosphine gas, which interferes with cell respiration. The rodenticide may be used to control many species of rodents, including mice, ground squirrels, prairie dogs, voles, moles, rats, muskrats, nutria and gophers. It may be used as an indoor or outdoor spot treatment for rodents as well as around burrows or underground in orchards, vineyards, various food crops, range lands, and non-crop areas. Zinc phosphide is formulated as a bait/solid, dust, granular, pellet/ tablet or wettable powder and is also applied as a broadcast treatment by ground or aerial applications.
Trade name
BAKER BRAND®[C]; BLUE-OX®; E-Z FLO®[C]; GOPHA-RID®; HOPKINS®; KILRAT®; MOLETOX II®; MOUS-CON®; MR. KILL RAT®; MR RAT GUARD®; NOTT ZINC PHOSPHIDE 93®; RATOL®; ROBAN II AG®[C]; RUMETAN®; ZINC- TOX®; ZP®
Safety Profile
Human poison by ingestion causing nausea, vomiting, death. Flammable when exposed to heat or flame. This material is stable while kept dry. In moist air, it decomposes slowly. Reacts violently with acids or acid fumes to emit the hghly toxic and flammable phosphine. Violent reaction with concentrated sulfuric acid, nitric acid, and oxidzing materials. Incompatible with HCl, H2SO4. When heated to decomposition it emits toxic fumes of POx and ZnO. Used as an acute rodenticide. See also PHOSPHIDES and ZINC COMPOUNDS.
Potential Exposure
It is used as an acute single feeding rodenticide.
Shipping
UN1714 Zinc phosphide, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material, 6.1-Poisono us materials.
Incompatibilities
Dust may form explosive mixture with air. Heat and contact with water causes decomposition, producing toxic and flammable fumes of phosphorus, zinc oxides; and toxic and flammable phosphine gas. Reacts violently with strong acids, including nitric, hydrochloric acid or sulfuric acid with the evolution of spontaneously flammable phosphine gas. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact can cause fires or explosions. Keep away from alkaline materials, strong bases. carbon dioxide, halogenated agents.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. Vanadium pentoxide may be salvaged or disposed of in a sanitary landfill.
Properties of ZINC PHOSPHIDE
| Melting point: | 420°C |
| Boiling point: | 1100°C |
| Density | 4,55 g/cm3 |
| Flash point: | 1100°C |
| storage temp. | Room Temperature, under inert atmosphere |
| solubility | reacts |
| solubility | insoluble in H2O, ethanol; reacacid; soluble in benzene |
| form | Pieces |
| appearance | gray to black crystals or powder |
| color | Dark Grey |
| Water Solubility | Insoluble in water and ethanol. Soluble in benzene. |
| Sensitive | Moisture Sensitive |
| Merck | 14,10152 |
| CAS DataBase Reference | 1314-84-7(CAS DataBase Reference) |
| EPA Substance Registry System | Zinc phosphide (1314-84-7) |
Safety information for ZINC PHOSPHIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H300:Acute toxicity,oral H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P231+P232:Handle under inert gas. Protect from moisture. P405:Store locked up. |
Computed Descriptors for ZINC PHOSPHIDE
Abamectin manufacturer
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium 3-Bromopyrazole (3aR,4R,5R,6aS)-hexahydro-5-Triethyl silyloxy-4-((E)-3-oxo-5-phenylpent-1- enyl)cyclopenta[b]furan-2-one. 1-Chlorocarbonyl-4-piperidinopiperidine 1-Bromo-4-phenyl-2-Butanone 4-Amino-2-fluoro-N-methylbenzamide 1,1'-Carbonyldiimidazole SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTIONRelated products of tetrahydrofuran
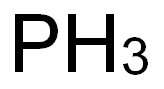
You may like
-
 Zinc phosphide CAS 1314-84-7View Details
Zinc phosphide CAS 1314-84-7View Details
1314-84-7 -
 Zinc phosphide CAS 1314-84-7View Details
Zinc phosphide CAS 1314-84-7View Details
1314-84-7 -
 Trizinc Diphosphide CAS 1314-84-7View Details
Trizinc Diphosphide CAS 1314-84-7View Details
1314-84-7 -
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate 99%View Details
54094-19-8 -
 85-81-4 99%View Details
85-81-4 99%View Details
85-81-4 -
![208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 (3aR,4R,5R,6aS)-5-(Benzoyloxy)hexahydro-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten- 1-yl]-2H-cyclopenta[b]furan-2-one 99%View Details
208111-98-2 -
 2033-24-1 99%View Details
2033-24-1 99%View Details
2033-24-1 -
 Meldrums acid 2033-24-1 99%View Details
Meldrums acid 2033-24-1 99%View Details
2033-24-1