Pentachlorophenol
Synonym(s):CLNK;Cytokine-dependent hematopoietic cell linker;Mast cell immunoreceptor signal transducer;MIST
- CAS NO.:87-86-5
- Empirical Formula: C6HCl5O
- Molecular Weight: 266.34
- MDL number: MFCD00002162
- EINECS: 201-778-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
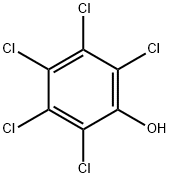
What is Pentachlorophenol?
Description
Pentachlorophenol is an old-line pest control agent that came under regulatory pressure decades ago. Beginning in the 1930s, it was used as an insecticide (including for termite control), a defoliant for crops before they were harvested, a wood preservative (notably in telephone poles), and a biocide with numerous other agricultural and industrial uses.
Pentachlorophenol is made by chlorinating phenol at high temperature. In the commercial process, chlorination is incomplete, and the product contains even more toxic impurities such as dibenzo-p-dioxin derivatives. The compound’s odor is similar to that of benzene or phenol; it is sparingly soluble in water and slow to decompose in nature.
Pentachlorophenol’s pervasive toxicity and environmental threats (see the hazard information table) doomed it to severe use restrictions by the 1980s. Today, wood preservation is the only use permitted by the US Environmental Protection Agency. Earlier this year, the EPA proposed to ban it completely. The comment period for this proposal expired in May, and EPA is currently proceeding with canceling the registration
For additional information, see EPA’s information page on pentachlorophenol.
Description
Pentachlorophenol is a restricted use pesticide and is used industrially as a wood preservative for utility poles, railroad ties, and wharf pilings. Pentachlorophenol was widely used as wood preservative until 1987 when its use was restricted to certified applicators. Pentachlorophenol is considered a probable human carcinogen and exposure to high levels can also have other health risks.
Pentachlorophenol is a synthetic substance, made from other chemicals, and does not occur naturally in the environment. It is made by only one company in the United States. At one time, it was one of the most widely used biocides in the United States. Since 1984, the purchase and use of pentachlorophenol has been restricted to certified applicators. It is no longer available to the general public. Application of pentachlorophenol in the home as an herbicide and pesticide accounted for only 3% of its consumption in the 1970s. Before use restrictions, pentachlorophenol was widely used as a wood preservative. It is now used industrially as a wood preservative for power line poles, cross arms, fence posts, and the like. Pure pentachlorophenol exists as colorless crystals. It has a very sharp characteristic phenolic smell when hot but very little odor at room temperature. Most people can begin to smell pentachlorophenol in water at less than 12 parts pentachlorophenol per million parts of water (ppm). Impure pentachlorophenol (the form usually found at hazardous waste sites) is dark gray to brown and exists as dust, beads, or flakes. Pentachlorophenol can be found in two forms: pentachlorophenol itself or as the sodium salt of pentachlorophenol. The sodium salt dissolves easily in water, but pentachlorophenol does not. These two forms have some different physical properties, but are expected to have similar toxic effects. Humans are generally exposed to technical-grade pentachlorophenol, which usually contains such toxic impurities as polychlorinated dibenzo- p-dioxins and dibenzofurans.
Chemical properties
Pentachlorophenol is a colorless to white, crystalline solid. It has a benzene-like odor; pungent when hot. The Odor Threshold in water is 1600 μg/L and the taste threshold in water is 30 μg/L.
Physical properties
White flakes or needles with a phenolic odor. At 40 °C, the average odor threshold concentration and the lowest concentration at which an odor was detected were 23 and 9.3 μg/L, respectively. At 25 °C, the lowest concentration at which a taste was detected was 8 μg/L (Young et al., 1996).
The Uses of Pentachlorophenol
Pentachlorophenol (PCP) is an odourless, white or light brown powder or crystal in appearance. It is used as herbicide and fungicide. Pentachlorophenol is incompatible with strong oxidising agents. Pentachlorophenol has a very sharp characteristic phenolic smell when hot but very little odour at room temperature. Pentachlorophenol is a synthetic substance made from other chemicals and does not occur naturally in the environment. Initially pentachlorophenol was widely used as a wood preservative. It is now used industrially as a wood preservative for power line poles, cross arms, fence post, etc.
Used as insecticide for terminate control; pre-harvest defoliant; general herbicide. Antimicrobial preservative and fungicide for wood, wood products, starches, textiles, paints, adhesives, leather, pulp, paper, industrial waste systems, building materials. Surface disinfectant.
The Uses of Pentachlorophenol
.Insecticide; fungicide; herbicide.
The Uses of Pentachlorophenol
Pentachlorophenol (PCP) is used for termite control, asa defoliant, and in the preservation of wood and wood products. It is an indoor air pollutant. It has been detected in timbers in the ppm range, causing contamination of air, surfaces, and materials in the homes. Its concentrations in blood samples have been reported in the range of sub-ppb to 110 μ/kg (Ruh et al. 1984). It has been detected in flue gas at 760–870°C (1400–1598°F) exit temperature from an incinerator at a concentration of 1.033 mg/m3 (Guinivan et al. 1985). The incinerator burned pentachlorophenol-treated wooden ammunition boxes and there was no afterburning. Methyl ethers of pentachlorophenol—pentachloroanisole and tetrachlorohydroquinone dimethyl ether —formed from microbial methylation of pentachlorophenol have been identified in the pg/m3 range in marine air samples from both the northern and southern hemispheres (Atlas et al. 1986)..
The Uses of Pentachlorophenol
Pentachlorophenol is used to control termites and, as the laurate ester, wood boring insects. The ester and the sodium salt are used to protect wood from fungal rot and as general herbicides and defoliants. The sodium salt is also used as a general disinfectant.
Definition
ChEBI: A chlorophenol that is phenol substituted by 5 chloro groups.
Production Methods
Pentachlorophenol can be produced by the chlorination of phenol in the presence of AlCl3, or by hydrolysis of hexachlorobenzene with NaOH in methanol.
General Description
A white crystalline solid. Slightly soluble in water. Noncombustible. Toxic by inhalation, ingestion, and skin absorption. Used as a fungicide and as a wood preservative.
Air & Water Reactions
Slightly soluble in water.
Reactivity Profile
Pentachlorophenol may react with strong oxidizing agents. Incompatible with strong bases, acid chlorides and acid anhydrides. Forms salts with alkaline metals. Solutions in oil cause natural rubber to deteriorate, but synthetic rubber may be used in equipment and for protective clothing .
Health Hazard
Dust or vapor irritates skin and mucous membranes, causing coughing and sneezing. Ingestion causes loss of appetite, respiratory difficulties, anesthesia, sweating, coma. Overexposure can cause death.
Health Hazard
Pentachlorophenol is a severe acute toxicant by ingestion and dermal penetration. The compound and its alkali salts can produce local and systemic effects. The symptoms of acute toxicity are headache, dizziness, sweating, nausea, vomiting, dyspnea, chest pain, weakness, fever, collapse, convulsions, and heart failure. Inhalation of its dusts or vapors can cause irritation of the eyes, nose, and throat, and coughing and sneezing. There is no evidence of chronic poisoning or any cumulative effects.
LD50 value, oral (mice): 117 mg/kg
LD50 value, skin (rats): 96 mg/kg Subacute toxicity studies on rats orally administered pentachlorophenol at a dose of 0.2 mmol/kg/day for 28 days showed no effect on growth. However, this treatment induced cell alterations in liver and changes in relative liver weights (Renner et al. 1987).Fathead minnows exposed to 8–130 g/L of pentachlorophenol for 90 days experienced no adverse effects on their survival, growth, or bone development (Hamilton et al. 1986). Fathead minnows exposed to 8–130 g/L of pentachlorophenol for 90 days experienced no adverse effects on their survival, growth, or bone development (Hamilton et al. 1986). McKim and associates (1986) have conducted aquatic toxicokinetic studies using 14C-labeled pentachlorophenol in rainbow trouts. At sublethal doses and over its 65hour half-life period, about 50% was eliminated over the gills, 30% in the feces and bile, and 20% in the urine. It was found that pentachlorophenol and its metabolites were rapidly eliminated from the bodies of fish .McKim and associates (1986) have conducted aquatic toxicokinetic studies using 14C-labeled pentachlorophenol in rainbow trouts. At sublethal doses and over its 65hour half-life period, about 50% was eliminated over the gills, 30% in the feces and bile, and 20% in the urine. It was found that pentachlorophenol and its metabolites were rapidly eliminated from the bodies of fish.
Fire Hazard
Special Hazards of Combustion Products: Generates toxic and irritating vapors.
Agricultural Uses
Fungicide, Herbicide, Slimicide, Wood preservative: Pentachlorophenol (PCP) is a commercially produced insecticide, fungicide, and slimicide. Since 1984 it has been restricted to certified applicators and is no longer available to the general public. It is primarily used to protect timber from fungal rot and wood-boring insects, but may also be used as a pre-harvest defoliant in cotton, a general pre-emergence herbicide, and as a biocide in industrial water systems. Not approved for use in EU countries. Not registered for use in the U.S. There are 48 global suppliers.
Trade name
(The U.S. EPA lists 626 active and canceled/ transferredlabelsforthischemical) CHEM-TOL®; CHLON®; CHLOROPHEN®; CRYPTOGIL OL®; DOWCIDE® 7; DOWICIDE® 7; DOW PENTACHLOROPHENOL DP-2 ANTIMICROBIAL®; DURA TREET II®; DUROTOX®; EP 30®; FORPEN-50®; FUNGIFEN®; GLAZDPENTA ®; GRUNDIER ARBEZOL®; LAUXTOL®; LIROPREM®; ONTRACK WE HERBICIDE®; ORTHO TRIOX®; OSMOSE WPC®; PENTACHLOROPHENOL, DOWICIDE EC-7®; PENTACHLOROPHENOL, DP- 2®; PENTACON®; PENTA-KIL®; PENTA READY®; PENTASOL®; PENWAR®; PERATOX®; PERMACIDE®; PERMAGARD®; PERMASAN®; PERMATOX DP- 2®PERMATOX PENTA®; PERMITE®; POL NU®; PREVENTOL P®; PRILTOX®; SANTOBRITE®; SANTOPHEN®; SINITUHO®; TERM-I-TROL®; THOMPSON'S WOOD FIX®; WATERSHED WP®; WEEDONE®; WOODTREAT A®
Safety Profile
Confirmed human carcinogen with experimental tumorigenic data. Human poison by ingestion. Poison experimentally by ingestion, skin contact, intraperitoneal, and subcutaneous routes. An experimental teratogen. Other experimental reproductive effects. A skin irritant. Mutation data reported. Acute poisoning is marked by weakness with changes in respiration, blood pressure, and urinary output. Also causes dermatitis, convulsions, and collapse. Chronic exposure can cause liver and hdney injury. Dangerous; when heated to decomposition it emits highly toxic fumes of Cl-. See also CHLOROPHENOLS
Potential Exposure
Pentachlorophenol (PCP) is a commercially produced bactericide, fungicide, and slimicide used primarily for the preservation of wood, wood products; and other materials. As a chlorinated hydrocarbon, its biological properties have also resulted in its use as an herbicide, and molluscicide. Two groups can be expected to encounter the largest exposures. One involves the small number of employees involved in the manufacture of PCP. All of these are presently under industrial health surveillance programs. The second and larger group are the formulators and wood theaters. Exposure, hygiene and industrial health practices can be expected to vary from the small theaters to the larger companies. The principal use as a wood preservative results in both point source water contamination at manufacturing and wood preservation sites and, conceivably, nonpoint source water contamination through runoff wherever there are PCP-treated lumber products exposing PCP to soil
Carcinogenicity
The IARC has determined that there is limited evidence for carcinogenicity in humans and sufficient evidence of carcinogenicity in experimental animals.
Environmental Fate
Biological. Under aerobic conditions, microbes in estuarine water partially dechlorinated pentachlorophenol to trichlorophenol (Hwang et al., 1986). The disappearance of
pentachlorophenol was studied in four aquaria with and without mud under aerobic and
anaerobic conditions. Potential biological and/or chemical products identified include
pentachloroanisole, 2,3,4,5-, 2,3,4,6- and 2,3,5,6-tetrachlorophenol (Boyle et al.,
Pentachlorophenol degraded in anaerobic sludge to 3,4,5-trichlorophenol which was
further reduced to 3,5-dichlorophenol (Mikesell and Boyd, 1985). In activated sludge, only
0.2% of the applied amount was mineralized to carbon dioxide after 5 days (Freitag
Pentachlorophenol was statically incubated in the dark at 25°C with yeast extract and
settled domestic wastewater inoculum. Significant biooxidation was observed but with a
gradual adaptation over a 14-day period to achieve complete degradation at 5 mg/L
substrate cultures. At a concentration of 10 mg/L, it took 28 days for pentachlorophenol
to degrade completely (Tabak et al., 1981).
Melcer and Bedford (1988) studied the fate of pentachlorophenol in municipal activated
sludge reactor systems that were operated at solids retention times of 10 to 20 days and
hydraulic retention times of 120 days. Under these conditions, pentachloropheno
Metabolic pathway
The insecticidal, antimicrobial and fungicidal properties of pentachlorophenol
were discovered some time ago and the compound was first
used in the 1930s for wood preservation and treatment. This and various
industrial uses and its herbicidal and molluscicidal properties have led to
its widespread use. Many countries have banned the use of pentachlorophenol
as a wood preservative. Its main uses are now in cooling towers,
paper mills and drilling muds (Litchfield and Rao, 1998). The compound
has become distributed in various ecosystems, including those close to
man’s living space. It is volatile and it may be absorbed via ingestion,
inhalation or skin contact.
There exists a very large literature on the toxicology, metabolism, persistence
and environmental effects and fate of pentachlorophenol, with
well over 500 papers published in the last 30 years. Pentachlorophenol is
rapidly and completely decomposed in sunlight; it is biodegraded in soil
and plants and it is metabolised in animals. Pathways include dechlorination,
methylation, oxidation, conjugation with sugars and sulfate and
ring scission. The environmental fate and metabolism of pentachlorophenol
were reviewed in 1986 by Engelhardt et al. (1986) and Renner and
Muecke (1986). The pathways reported below are largely taken from these
papers which are supported by more than 120 references. Other selected
papers which cover important aspects are also quoted. The microbial
degradation of the compound, particularly in relation to waste clean-up,
has been reviewed recently (Litchfield and Rao, 1998).
Metabolism
Pentachlorophenol was metabolized in rats by conjugation with glucuronic acid and eliminated as the glucuronide. P450 catalyzed oxidative dechlorination also occurred to form tetrachlorohydroquinone, and this was conjugated to form a monoglucuronide representing 27% of the dose administered. Other metabolites have been reported, including isomeric tetrachlorophenols, tetrachlorocatechol and tetrachlororesorcinol. Trace amounts of benzoquinones were also noted. Metabolites in female rats were tetrachloromonophenols, diphenols, and hydroquinones.
Solubility in organics
At 20 °C (g/100 g solution): methanol (57.0), anhydrous ethanol (53.0), 95% ethanol (47.5), diethylene glycol monomethyl ether (48.0), pine oil (32.0), diethylene glycol monoethyl ether (30.0), diethylene glycol (27.5), 2-ethoxyethanol (27.0), dioxane (11.5), benzene (11.0), ethylene glycol (6.0), diesel oil (3.1), fuel oil (2.6) (Carswell and Nason, 1938).
Solubility in water
At 20 °C (g/100 g solution): methanol (57.0), anhydrous ethanol (53.0), 95% ethanol (47.5), diethylene glycol monomethyl ether (48.0), pine oil (32.0), diethylene glycol monoethyl ether (30.0), diethylene glycol (27.5), 2-ethoxyethanol (27.0), dioxane (11.5), benzene (11.0), ethylene glycol (6.0), diesel oil (3.1), fuel oil (2.6) (Carswell and Nason, 1938).
Shipping
UN3155 Pentachlorophenol, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Crystallise it twice from toluene/EtOH. Sublime it in vacuo.[Beilstein 6 IV 1025.]
Degradation
Pentachlorophenol has the typical weak acidic properties of a phenol,
readily forming the sodium salt. At physiological pH a major proportion
is ionised and the metabolism (but not necessarily the mobility and
absorption) of pentachlorophenol and its sodium salt should be very similar.
The laurate ester, being lipophilic, is absorbed more readily than the
phenate ion and it is also more volatile. However, the ester should be
readily hydrolysed in dilute base to pentachlorophenol and lauric acid
and by estersases in vivo to the same products. Thus the metabolism of the
three forms may be considered together.
Pentachlorophenol is rapidly degraded under conditions of aqueous
photolysis in W light and sunlight (Engelhardt et al., 1986). Products
detected (Scheme 1) include the reductive dechlorination products
2,3,4,6- and 2,3,5,6-tetrachlorophenol (2 and 3) and trichlorophenols.
Ring chlorine atoms were displaced by hydroxyl groups to afford
2,3,5,6-tetrachlorohydroquinone (4), tetrachlorocatechol (5) and tetrachlororesorcinol(
6). The hydroquinone (4) was very rapidly decomposed in air. Irradiation of each of 4, 5 and 6 afforded trichlorobenzenediols,
trichloroquinones and 2,3-dichloromaleic acid (7).
Hydroquinone 4 oxidised in the dark (and in light) to 2,3,5,6-
tetrachloro-l,4-benzoquinone (8), the 2-hydroxy analogue (9), the
dichlorohydroxybenzoquinone (10) and the maleic acid (7). The latter
eventually affords CO2 and HCl.
Exposure of an aqueous solution of the sodium salt to sunlight gave
small amounts of octachlorodibenzodioxin but none of the extremely
toxic 2,3,7,8-tetrachloro derivative could be detected. Much of the original
work on the photolysis of pentachlorophenol was reported by Wong and
Crosby (1978).
Toxicity evaluation
The toxicology has been addressed in a
recent risk assessment (119). Acutely, pentachlorophenol
was reported to have LD50 values in the rat of 12 mg/kg (inhalation) and 146 mg/kg (M)–175 mg/kg (F) by oral
gavage. More detailed studies of the toxicology of pentachlorophenol
have been compromised by the toxicity of
impurities present in most of the earlier samples used
in the evaluation process.Although a number of toxicity
studies have been conducted with both known impurities
and TCHQ, it is often difficult to know whether
animal experiments are valid for human health risk assessment.
Nevertheless, it appears that the main target organ
of purified TCP in animals is the liver.
This toxicity
was manifested as liver inflammation, increased relative
weight, and increased serum alkaline phosphatase. The
estimated chronic NOEL in the dog for these effects was
0.15 mg/kg/day, from a 1-year study, based on a LOEL of 1.5 mg/kg/day. In the rat, a significantly increased
incidence of mesotheliomas (p<0.05) and nasal carcinomas
in males was reported at the highest dose tested,
~60 mg/kg/day.
Incompatibilities
Reacts violently with strong oxidizers, acids, alkalies, and water.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Incineration (600°to 900°C) coupled with acequate scrubbing and ash disposal facilities. Alternatively pentachlorophenol
Properties of Pentachlorophenol
| Melting point: | 165-180 °C(lit.) |
| Boiling point: | 310 °C(lit.) |
| Density | 1.978 g/mL at 25 °C(lit.) |
| vapor density | 9.2 (vs air) |
| vapor pressure | 40 mm Hg ( 211.2 °C) |
| refractive index | 1.6310 (estimate) |
| Flash point: | 11 °C |
| storage temp. | 0-6°C |
| solubility | 14 mg/l |
| solubility | Chloroform (Slightly), Ethyl Acetate (Sparingly) |
| pka | 4.80 (Blackman et al., 1955) 5.3 (Eder and Weber, 1980) |
| form | Liquid |
| Water Solubility | 80 mg l-1(30 °C) |
| appearance | white crystals or powder |
| Specific Gravity | 1.979 |
| Merck | 7109 |
| BRN | 1285380 |
| Henry's Law Constant | 21 (quoted, Petrasek et al., 1983) |
| Exposure limits | NIOSH REL: IDLH 0.5 mg/m3, IDLH 2.5 mg/m3; OSHA PEL: TWA
0.5 mg/m3; ACGIH TLV: TWA 0.5 mg/m3 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 87-86-5(CAS DataBase Reference) |
| IARC | 1 (Vol. 53, 71, 117) 2019 |
| EPA Substance Registry System | Pentachlorophenol (87-86-5) |
Safety information for Pentachlorophenol
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H330:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H351:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pentachlorophenol
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
