CALCIUM PHOSPHIDE
- CAS NO.:1305-99-3
- Empirical Formula: Ca3P2
- Molecular Weight: 182.18
- MDL number: MFCD00015986
- EINECS: 215-142-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
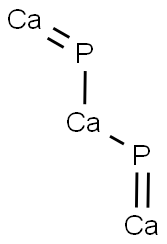
What is CALCIUM PHOSPHIDE?
Description
Calcium phosphide has the molecular formula of
Ca3P2 and the molecular weight of 182.1825 g/mol. Its
CAS number is 1305-99-3. It is a red-brown crystalline
material with a melting point of 1605°C. Its density is
2.51 g/cm3. It readily reacts with water to form phosphine,
PH3, but is insoluble in ethanol.
It is easily prepared by reacting the metal with red
phosphorus at high temperature. The best way is to
sublime the P4 at 450°C in an inert gas stream and react
it with Mg metal at 750°C:
6Ca + 2P4?2Ca3P4
Chemical properties
Calcium phosphide is a gray granular solid or reddish-brown crystalline solid. It has a musty odor, somewhat like acetylene.
The Uses of CALCIUM PHOSPHIDE
Metal phosphides, primarily Ca3P2, have been used as rodenticides. Calcium phosphide baits have strong, pungent garlic-like odor characteristic for phosphine liberated by hydrolysis. The odor attracts rodents, but has a repulsive effect on other animals, who are not receptive to the smell. This salt has uses in incendiary bombs and other explosives. On contact with acids or water, calcium phosphide releases phosphine, which ignites spontaneously. It is also used in fireworks, torpedoes, self-igniting naval pyrotechnic flares, and various water-activated ammunition.
The Uses of CALCIUM PHOSPHIDE
For signal fires; in purification of Cu and Cu alloys; as rodenticide.
General Description
CALCIUM PHOSPHIDE appears as red-brown crystals to gray granular lumps. CALCIUM PHOSPHIDE reacts with water to form calcium hydroxide and phosphine, a flammable poisonous gas. Phosphine will normally ignite spontaneously in contact with air. If there is an excess of water this fire of phosphine will not normally ignite surrounding combustible material.
Reactivity Profile
CALCIUM PHOSPHIDE and hydrochloric acid undergo a very energetic reaction [Mellor 8:841 1946-47]. Calcium and other alkaline earth phosphides incandesce in oxygen when heated.
Hazard
Dangerous fire risk; decomposed by water to phosphine, which is highly toxic and flammable. See phosphine.
Health Hazard
Inhalation or ingestion causes faintness, weakness, nausea, vomiting. External contact with dust causes irritation of eyes and skin.
Fire Hazard
Behavior in Fire: Can cause spontaneous ignition if wet. Contributes dense smoke of phosphoric acid.
Safety Profile
Highly toxic due to phosphde, which in presence of moisture emits phosphine. The phosphine may ignite spontaneously in air. Incandescent reaction with oxygen at 300°C. Incompatible with dichlorine oxide. When heated to decomposition it emits toxic fumes of POx. See also CALCIUM COMPOUNDS and PHOSPHIDES.
Potential Exposure
A strong reducing agent. Forms spontaneously combustible phosphine gas in moist air. Contact with water or acids release phosphine gas, and can cause explosions. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, chlorine monoxide, halogens, halogen acids, oxygen, sulfur
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
storage
Color Code—Yellow Stripe (strong reducingagent): Reactivity Hazard; Store separately in an area isolated from flammables, combustibles, or other yellow-codedmaterials. Store in tightly closed containers in a cool,well-ventilated area away from strong oxidizers (such aschlorine, bromine, and fluorine), strong acids (such ashydrochloric, sulfuric, and nitric), oxygen, sulfur, or moisture since violent reactions occur. Sources of ignition, suchas smoking and open flames, are prohibited where Calciumphosphide is handled, used, or stored. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of Calcium phosphide. Wherever Calciumphosphide is used, handled, manufactured, or stored, useexplosion-proof electrical equipment and fittings. Do notstore large amounts of this material in a room protected bywater sprinkler systems. Protect containers against physicaldamage.
Shipping
UN1360 Calcium phosphide, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet material
Incompatibilities
A strong reducing agent. Forms spontaneously combustible phosphine gas in moist air. Contact with water or acids release phosphine gas, and can cause explosions. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, chlorine monoxide, halogens, halogen acids, oxygen, sulfur
Waste Disposal
Disposal of unused product must be undertaken by qualified personnel who are knowledgeable in all applicable regulations and follow all pertinent safety precautions including the use of appropriate protective equipment. For proper handling and disposal, always comply with federal, state, and local regulations
Properties of CALCIUM PHOSPHIDE
| Melting point: | 1600°C |
| Density | 2,51 g/cm3 |
| solubility | reacts with H2O; insoluble in ethanol,ethyl ether |
| form | granular |
| color | red-brown hygroscopic crystals, crystalline |
| Water Solubility | decomposes in H2O to form flammable phosphine [MER06]; insoluble alcohol, ether [HAW93] |
| Sensitive | Moisture Sensitive |
| Merck | 14,1695 |
| EPA Substance Registry System | Calcium phosphide (1305-99-3) |
Safety information for CALCIUM PHOSPHIDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H300:Acute toxicity,oral H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P231+P232:Handle under inert gas. Protect from moisture. P370+P378:In case of fire: Use … for extinction. P422:Store contents under … |
Computed Descriptors for CALCIUM PHOSPHIDE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8