Zinc chloride
Synonym(s):Additive Screening Solution 29/Kit-No 78374;Dichlorozinc;Zinc chloride;Zinc chloride solution;Zinc dichloride
- CAS NO.:7646-85-7
- Empirical Formula: Cl2Zn
- Molecular Weight: 136.3
- MDL number: MFCD00011295
- EINECS: 231-592-0
- Update Date: 2025-12-17 09:49:47
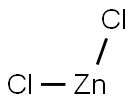
What is Zinc chloride?
Absorption
Zinc is approximately 33% orally bioavailable in humans but bioavailability can vary between patients and depending on current zinc levels. Further data regarding the pharmacokinetics of zinc chloride are not readily available.
Toxicity
Patients experiencing and overdose of Zinc chloride may present with hypotension, pulmonary edema, diarrhea, vomiting, jaundice, and oligouria. The oral LD50 in mice is 329 mg/kg and in rats is 350 mg/kg.
Background
Zinc chloride is a solution of ions indicated for use in total parenteral nutrition to maintain zinc levels and prevent deficiency syndromes.
Zinc chloride was granted FDA approval before 26 June 1986.
Indications
Zinc chloride is indicated for use total parenteral nutrition to maintain zinc serum levels and prevent deficiency syndromes.
Pharmacokinetics
Zinc is a cofactor in many enzymes and mediates a number of catalytic, structural, and regulatory roles in the body. It has a wide therapeutic index and long duration of action, as most zinc in the body is reabsorbed. Patients should be counselled regarding the risk of administration in patients with severe kidney dysfunction.
Hazards
Zinc chloride is caustic. Zinc chloride intended for use as a pesticide apparently has not led to injury other than dermatitis. Ingestion undoubtedly would produce illness similar to that caused by copper sulfate. In several instances, the preparation or storage of an acid food in a galvanized or zinc-plated vessel has led to severe vomiting and to headache and discomfort in the chest (Hegsted et al., 1945).
Metabolism
Zinc chloride dissociates into ions in vivo and does not undergo further metabolism.
The Uses of Zinc chloride
Zinc chloride is strongly deliquescent (water-absorbing) and is utilized as a drying agent and as a flux. In aqueous solution it is used as a wood preservative.
Preparation
zinc chloride is prepared from the reaction of hydrochloric acid with zinc metal or zinc oxide.
Properties of Zinc chloride
| Melting point: | 293 °C (lit.) |
| Boiling point: | 732 °C (lit.) |
| Density | 1.01 g/mL at 20 °C |
| vapor pressure | 1 mm Hg ( 428 °C) |
| Flash point: | 732°C |
| storage temp. | 2-8°C |
| solubility | H2O: 4 M at 20 °C, clear, colorless |
| appearance | White crystalline solid |
| form | crystalline |
| pka | pKa 6.06 (Uncertain) |
| Specific Gravity | 2.91 |
| color | white |
| PH | 5 (100g/l, H2O, 20℃) |
| Odor | wh. cubic cryst., odorless |
| Water Solubility | 432 g/100 mL (25 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,10132 |
| Exposure limits | ACGIH: TWA 1 mg/m3; STEL 2 mg/m3 OSHA: TWA 1 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 1 mg/m3; STEL 2 mg/m3 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 7646-85-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Zinc dichloride(7646-85-7) |
| EPA Substance Registry System | Zinc chloride (7646-85-7) |
Safety information for Zinc chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Zinc chloride
| InChIKey | JIAARYAFYJHUJI-UHFFFAOYSA-L |
Zinc chloride manufacturer
Sigma Chemical Industries
Sainor Laboratories Pvt Ltd Unit III
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Zinc Chloride 99%View Details
Zinc Chloride 99%View Details -
 Zinc Chloride 99%View Details
Zinc Chloride 99%View Details -
 Zinc chloride 98%View Details
Zinc chloride 98%View Details -
 Zinc chloride 98%View Details
Zinc chloride 98%View Details -
 Zinc chloride CAS 7646-85-7View Details
Zinc chloride CAS 7646-85-7View Details
7646-85-7 -
 Zinc chloride CAS 7646-85-7View Details
Zinc chloride CAS 7646-85-7View Details
7646-85-7 -
 Zinc chloride hydrate CAS 7646-85-7View Details
Zinc chloride hydrate CAS 7646-85-7View Details
7646-85-7 -
 Zinc chloride, 95% 99%View Details
Zinc chloride, 95% 99%View Details
