7646-85-7
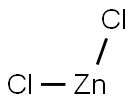
Product Name:
Zinc chloride
Formula:
Cl2Zn
Synonyms:
Additive Screening Solution 29/Kit-No 78374;Dichlorozinc;Zinc chloride;Zinc chloride solution;Zinc dichloride
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | White, very deliquesce granules, or fused pieces or rods |
|---|---|
| Odor | Odorless |
| Boiling Point | 1350 °F at 760 mmHg (NIOSH, 2023) |
| Melting Point | 554 °F (NIOSH, 2023) |
| Solubility | 435 % at 70 °F (NIOSH, 2023) |
| Density | 2.91 at 77 °F (NIOSH, 2023) - Denser than water; will sink |
| Vapor Pressure | 0 mmHg (approx) (NIOSH, 2023) |
| Stability/Shelf Life | In weak aqueous solution has tendency to form insoluable basic salt by hydrolysis; about 1/2 its weight of ammonium chloride has been used for stabilization |
| Autoignition Temperature | Not flammable (USCG, 1999) |
| Decomposition | When heated to decomposition it emits toxic fumes of /hydrogen chloride and zinc oxide/. |
| Corrosivity | Fume corrosive to metals /zinc chloride fumes/ |
| pH | Aqueous solutions of zinc chloride are acidic (pH = 1.0 for 6 M) |
| Refractive Index | INDEX OF REFRACTION: 1.681; 1.713 |
| Other Experimental Properties | With much water, some zinc oxylchloride is formed |
| Chemical Classes | Metals -> Metals, Inorganic Compounds |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H314:Skin corrosion/irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 136.3 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 133.866847 g/mol |
| Monoisotopic Mass | 133.866847 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 2.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Zinc chloride is a white crystalline solid. It is soluble in water. It is corrosive to metals and therefore irritating to the skin, eyes and mucous membranes. It is used for preserving wood, in soldering fluxes, as a catalyst in chemical metals and manufacturing, and for many other uses.
RELATED SUPPLIERS
All suppliers(21)
