Zileuton
Synonym(s):(±)-N-hydroxy-N-(1-benzo[b]thien-2-ylethyl)urea;Zileuton
- CAS NO.:111406-87-2
- Empirical Formula: C11H12N2O2S
- Molecular Weight: 236.29
- MDL number: MFCD00866097
- EINECS: 601-087-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
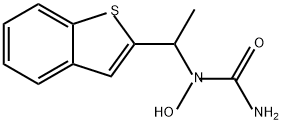
What is Zileuton?
Absorption
Rapidly and almost completely absorbed. The absolute bioavailability is unknown.
Toxicity
Minimum oral lethal dose of zileuton in various preparations was 500-4000 mg/kg in mice and 300-1000 mg/kg in rats (providing greater than 3 and 9 times the systemic exposure [AUC] achieved at the maximum recommended human daily oral dose, respectively).
Description
Zileuton is a reversible 5-lipoxygenase (5-LO) inhibitor. It inhibits 5-LO activity in rat basophilic leukemia-1 (RBL-1) cell supernatant with an IC50 value of 0.5 μM. Zileuton inhibits leukotriene B4 (LTB4; ) production induced by the calcium ionophore A23187 in purified human peripheral blood polymorphonuclear leukocytes (PMNLs; IC50 = 0.6 μM). Zileuton (10 mg/kg, p.o.) prevents antigen challenge-induced increases in specific lung resistance in a sheep model of asthma at 4 to 8 hours post-challenge following administration 2 hours pre-challenge. Formulations containing zileuton have been used in the prophylaxis and chronic treatment of asthma.
Description
Zyflo was launched in the US for chronic asthma. It can be prepared in three steps from 2-acetylbenzo[b]thiophene. Zyflo is a reversible direct inhibitor of 5- lipoxgenase that is orally-active. It was able to effect a 70-100 YO reduction in LTB4, LTE4, LTD4 and LTC4. Zyflo has no effect on myeloperoxidase activity, neutrophil degranulation, mast cell histamine release or phospholipase A2 activities. It did not inhibit cyclooxygenase as witnessed by the formation of TXB2. It significantly attentuated asthmatic response to cold dry air, inhibited exercise-induce bronchoconstriction, and attenuated induced bronchospasms. Zyflo has antiinflammatory effects as witnessed by a decrease in edema, mucus production and cellular infiltration. It had a bronchodilatory effect within 2 h and increased spirometry results by 18%.
Chemical properties
Crystalline Solid
Originator
Abbott (US)
The Uses of Zileuton
An inhibitor of 5-lipoxygenase, the initial enzyme in the biosynthesis of leukotrienes from Arachidonic Acid. Used as an antiasthmatic
The Uses of Zileuton
gastric acid secretion inhibitor
The Uses of Zileuton
An inhibitor of 5-lipoxygenase, the initial enzyme in the biosynthesis of leukotrienes from Arachidonic Acid. Used as an antiasthmatic.
Background
Leukotrienes are substances that induce numerous biological effects including augmentation of neutrophil and eosinophil migration, neutrophil and monocyte aggregation, leukocyte adhesion, increased capillary permeability, and smooth muscle contraction. These effects contribute to inflammation, edema, mucus secretion, and bronchoconstriction in the airways of asthmatic patients. Zileuton relieves such symptoms through its selective inhibition of 5-lipoxygenase, the enzyme that catalyzes the formation of leukotrienes from arachidonic acid. Specifically, it inhibits leukotriene LTB4, LTC4, LTD4, and LTE4 formation. Both the R(+) and S(-) enantiomers are pharmacologically active as 5-lipoxygenase inhibitors in in vitro systems. The immediate release tablet of Zileuton has been withdrawn from the US market.
Indications
For the prophylaxis and chronic treatment of asthma in adults and children 12 years of age and older.
What are the applications of Application
Zileuton is a 5-LO (5-lipoxygenase) inhibitor
Definition
ChEBI: A member of the class of 1-benzothiophenes that is 1-benzothiophene in which the hydrogen at position 2 is replaced by a 1-[carbamoyl(hydroxy)amino]ethyl group. A selective 5-lipoxygenase inhibitor, it inhibits the formation of leukotrienes LTB4, LTC4, LDT , and LTE4. It is used for the management of chronic asthma.
Manufacturing Process
N-Hydroxy-N-(1-benzo[b]thien-2-ylethyl) acetamide
1. 2-Acetyl benzo[b]thiophene.
Method a. Benzo[b]thiophene (10 g, 75 mmole) was dissolved in THF (50 ml)
and cooled to -78°C. n-Butyl lithium (28 ml, 2.7 M in hexanes) was added.
The mixture was stirred for 15 minutes and N,O-dimethyl acetohydroxamic
acid was added. Following an additional 30 minutes of stirring, the reaction
was quenched at -78°C with ethanol and 2 N HCl solution and extracted into
ether. The solvent was removed in vacuo and the residue chromatographed on
silica gel eluting with 20% ether in pentane to yield 6.9 g of the desired
product as a white solid.
Method b. To a solution of benzo[b]thiophene (10.0 g, 75 mmole) in THF (50
ml) was added n-butyl lithium (33 ml, 2.5 M in hexanes) at -70°C under N 2 .
The mixture, containing a white precipitate, was stirred at 70°C for 1 hour.
Acetaldehyde (4.6 ml, 82 mmole) was added dropwise. After a few minutes
the reaction was quenched with saturated NH 4 Cl solution. The layers were
separated, the organic layer dried over MgSO4, filtered, and evaporated to
give a white solid (10 g) which was used directly for the next step.
The alcohol prepared as described above (1.0 g) in acetone (50 ml) was
cooled to 5°C and Jones Reagent was added dropwise until the orange yellow
color persisted (1.4 ml). The reaction mixture was diluted with water and the
desired product precipitated. It was collected by filtration to give 0.85 g.
2. 2-Acetyl benzo[b]thiophene oxime.
2-Acetyl benzo[b]thiophene (5 g, 28.4 mmole), prepared as described in step
1 above, and hydroxylamine hydrochloride (3.0 g, 42.6 mmole) were
dissolved in a mixture of ethanol (50 ml) and pyridine (50 ml) and allowed to
stir at room temperature for 2 hours. Most of the solvent was removed in
vacuo and the residue dissolved in ether. After washing with 2 N HCl (100 ml),
the solution was dried over MgSO 4 and evaporated. A white crystalline solid was obtained and was carried on without further purification. An alternative
work-up may also be used. The reaction mixture was diluted with water (300
ml) and the product precipitated. It was filtered off and dried in vacuo.
3. 1-Benzo[b]thien-2-ylethyl hydroxylamine. The oxime prepared as in step 2
above (3.5 g, 18.5 mmole) was dissolved in ethanol (25 ml) and cooled to
0°C. Borane pyridine complex (3.7 ml, 37 mmole) was added via syringe
under nitrogen followed 10 minutes later by 20% HCl in ethanol (30 ml).
Within 30 minutes the reaction was complete and was brought to pH 9 with
the addition of solid sodium carbonate or 2 N NaOH. The mixture was
extracted into ether and dried over MgSO 4 . After evaporation a white solid
(3.0 g) was obtained. This was carried on without further purification.
N-Hydroxy-N-(1-benzo[b]thien-2-ylethyl)urea
Method A. 1-Benzo[b]thien-2-yl ethyl hydroxyl amine prepared as described
above, step 3 (2.0 g, 10 mmole), was refluxed for 30 minutes with
trimethylsilyl isocyanate (1.65, 14.2 mmole) in dioxane (30 ml). The reaction
mixture was then washed with saturated NH 4 Cl solution, dried with MgSO 4 ,
and evaporated.
Method B. 1-Benzo[b]thien-2-yl ethyl hydroxyl amine prepared as described in
step 3, was dissolved in toluene (100 ml) and HCl gas was bubbled through
the mixture at a moderate rate for about 4 minutes. The solution was then
heated to reflux and phosgene was bubbled through for another 4 minutes.
After an additional one hour reflux, the mixture was allowed to cool to room
temperature and then added to excess cold ammonium hydroxide solution.
The precipitate was collected and recrystallized. Melting point: 157°-158°C.
NMR (300 MHz), and mass spectrum confirmed the structure of the prepared
compound.
brand name
Zyflo (Sensus).
Therapeutic Function
Antiallergic, Antiinflammatory
Biological Activity
Orally active 5-lipoxygenase (5-LOX) inhibitor that inhibits LTB 4 synthesis (IC 50 values are 0.56, 2.3 and 2.6 μ M in dog, rat and human blood respectively). Inhibits antigen-induced contraction of tracheal strips in vitro (IC 50 = 6 μ M) and exhibits antiasthmatic activity in vivo . Also weakly inhibits CYP1A2 (K i = 66 - 98 μ M).
Biochem/physiol Actions
Zileuton is an anti-asthmatic, an inhibitor of 5-lipoxygenase; the initial enzyme in the biosynthesis of leukotrienes from arachidonic acid.
Pharmacokinetics
Zileuton is an asthma drug that differs chemically and pharmacologically from other antiasthmatic agents. It blocks leukotriene synthesis by inhibiting 5-lipoxygenase, an enzyme of the eicosanoid synthesis pathway. Current data indicates that asthma is a chronic inflammatory disorder of the airways involving the production and activity of several endogenous inflammatory mediators, including leukotrienes. Sulfido-peptide leukotrienes (LTC4, LTD4, LTE4, also known as the slow-releasing substances of anaphylaxis) and LTB4, a chemoattractant for neutrophils and eosinophils, are derived from the initial unstable product of arachidonic acid metabolism, leukotriene A4 (LTA4), and can be measured in a number of biological fluids including bronchoalveolar lavage fluid (BALF) from asthmatic patients. In humans, pretreatment with zileuton attenuated bronchoconstriction caused by cold air challenge in patients with asthma.
Metabolism
Hepatic. Zileuton and its N-dehydroxylated metabolite are oxidatively metabolized by the cytochrome P450 isoenzymes 1A2, 2C9 and 3A4.
Storage
Store at +4°C
References
1) Carter et al. (1991), 5-Lipoxygense inhibitory activity of zileuton; J. Pharmacol. Exp. Ther. 256 929 2) Rossi et al. (2010), The 5-lipoxygenase inhibitor, zileuton, suppresses prostaglandin biosynthesis by inhibition of arachidonic acid release in macrophages; Br. J. Pharmacol. 161 555
Properties of Zileuton
| Melting point: | 157-158°C |
| Boiling point: | 449.4±47.0 °C(Predicted) |
| Density | 1.401±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | DMSO: ≥20mg/mL at ~60°C (warm up to 60 C for 5min) |
| pka | pKa 10.3(H2O
t undefined
I undefined) (Uncertain) |
| form | powder |
| color | white to off-white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 111406-87-2(CAS DataBase Reference) |
Safety information for Zileuton
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Zileuton
Zileuton manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 Zileuton 99%View Details
Zileuton 99%View Details -
 111406-87-2 98%View Details
111406-87-2 98%View Details
111406-87-2 -
 111406-87-2 98%View Details
111406-87-2 98%View Details
111406-87-2 -
 Zileuton 111406-87-2 98%View Details
Zileuton 111406-87-2 98%View Details
111406-87-2 -
 Zileuton 99%View Details
Zileuton 99%View Details -
 Zileuton 98% CAS 111406-87-2View Details
Zileuton 98% CAS 111406-87-2View Details
111406-87-2 -
 Zileuton CAS 111406-87-2View Details
Zileuton CAS 111406-87-2View Details
111406-87-2 -
 Zileuton CAS 111406-87-2View Details
Zileuton CAS 111406-87-2View Details
111406-87-2
