zidometacin
- CAS NO.:62851-43-8
- Empirical Formula: C19H16N4O4
- Molecular Weight: 0
- MDL number: MFCD00866045
- EINECS: 263-740-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
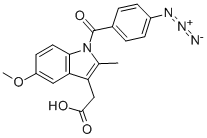
What is zidometacin?
Originator
Zidometacin,ZYF Pharm Chemical
The Uses of zidometacin
Antiinflammatory.
Manufacturing Process
A hot solution of 5.2 g (15.4 mmol) of 1-(p-aminobenzoyl)-5-methoxy-2-
methyl-3-indolylacetic acid in 65 ml of acetic acid is rapidly cooled to 25-30°C
(try to avoid crystallization). This cold solution and a solution of 1.145 g (16.6
mmol) of sodium nitrite in 40 ml of water are added simultaneously to 18 ml
of concentrated hydrochloric acid at -5°C with stirring. The resulting solution
(about 25°C) is red colored and on cooling to 0°C a crystalline solid begins to
separate. After 10 minutes at 0°C, an ice-cold solution of 1.057 g (16.25
mmol) of sodium azide in 40 ml of water is added in portions. A cream
colored precipitate forms immediately, accompanied by copious evolution of
nitrogen. The reaction is completed when no more red color is visible. If
necessary, a further little excess of NaN3 solution may be added. Stirring is
continued for 10 minutes at 0°C and then the mixture is extracted with
ethylacetate. The organic phase is separated, washed with water, dried over
Na 2 SO 4 and concentrated in vacuum to give 5.67 g (100%) of 1-(p-
azidobenzoyl)-5-methoxy-2-methyl-3-indolylacetic acid. Thin layer
chromatography on silica gel gives one spot in the system chloroform-ethanol 95:5. An analytical sample is obtained by crystallization from methanol-water:
MP: 170-172°C (with gas evolution). The structure of zidometacin is
confirmed by IR spectrum.
The starting compounds were synthesized next way:
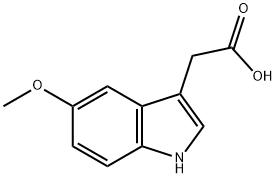
Preparation of methyl 1-(p-nitrobenzoyl)-5-methoxy-2-methyl-3-
indolylacetate:
To a solution of 23.3 g (0.1 mole) of methyl-5-methoxy-2-methyl-3-
indolylacetate in 50 ml of dry toluene are added 3 g of 80% sodium hydride.
The mixture is stirred at room temperature for 4 hours and then a solution of
18.56 g (0.1 mole) of p-nitrobenzoylchloride in 80 ml of dry toluene is added
slowly thereto over a 30-minute period. The reaction mixture is boiled for 30
hours. After cooling it is poured into 400 ml of ice-water and 15 ml of acetic
acid. The separated toluene solution is washed with a large quantity of water,
dried over sodium sulfate and evaporated to a syrup which is dissolved in
ether. Slow evaporation of this solution in an open beaker gives 10 g of
methyl-1-(p-nitrobenzoyl)-5-methoxy-2-methyl-3-indolylacetate as yellow
prisms. Another quantity may be recovered from the oily residue after
chromatography on a silica gel column (elution with benzene). MP: 134-135°C
(crystallization from MeOH).
Preparation of 1-(p-nitrobenzoyl)-5-methoxy-2-methyl-3-indolylacetic acid:
A solution of 4.9 g (12.8 mmol) of methyl-1-(p-nitrobenzoyl)-5-methoxy-2-
methyl-3-indolylacetate in 40 ml of acetic acid containing 400 mg of p-
toluene-sulfonic acid is refluxed for 20 hours and then concentrated in
vacuum. The gummy residue is extracted with ethyl acetate. The extract is
filtered from insoluble material, washed with water and dried over sodium
sulfate. Removal of the solvent under reduced pressure affords the desired
product as yellow crystals; MP: 185-186°C.
Preparation of 1-(p-aminobenzoyl)-5-methoxy-2-methyl-3-indolylacetic acid:
20 g (54.3 mmol) of 1-(p-nitrobenzoyl)-5-methoxy-2-methyl-3-indolylacetic
acid is dissolved in 1200 ml of hot methanol and hydrogenated in the
presence of 2.64 g of 10% palladium on charcoal as catalyst. After 164 mmol
of hydrogen have been consumed, the hydrogenation is stopped, and the
solution filtered to remove the catalyst. The filtrate is concentrated in vacuum
to give, in nearly theoretical yield, the title p-amino derivative. A
crystallization from methanol-water gave an analytical sample: MP 198-200°C
(dec.) crystals from MeOH-H 2 O.
Therapeutic Function
Antiinflammatory
Safety information for zidometacin
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8