Zepastine
- CAS NO.:28810-23-3
- Empirical Formula: C22H26N2O3S
- Molecular Weight: 398.524
- MDL number: MFCD00869033
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
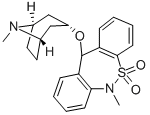
What is Zepastine?
Originator
Zepastine,ZYF Pharm Chemical
Manufacturing Process
5,11-Dihydro-11-methyl-5,10,10-trioxodibenzo[c,f][1,2]thiazepine 16 g is
suspended at 300 ml of methanol and treated with of 3 g of sodium
borohydride. The reaction mixture is kept at room temperature overnight,
heated to dissolve precipitated material, acidified with 10% acetic acid and
allowed to cool. The crystalline product is collected on a filter, washed with
water and recrystallized from isopropanol, MP: 138°C.
5-Chloro-5,11-dihydro-10,10-dioxo-11-methyldibenzo[c,f][1,2]thiazepine:
A portion of the above product, 5 g, is dissolved in 50 ml of benzene and the
solution is saturated with hydrogen chloride. External cooling is supplied in
order to maintain the temperature near room temperature. The product
crystallizes from the solution during this process. The mixture is kept at room
temperature for one hour and the product is collected on a filter, yield 5 g;
MP: 224-225°C. This material is purified by recrystallization from toluene, MP:
230°C.
3-(5,11-Dihydro-10,10-dioxo-11-methyldibenzo[c,f][1,2]thiazepin-5-
yloxy)tropane and the hydrogen maleate salt thereof. (This name was given
by the authors of U. S. Patent No 3 700 633. It corresponds to endo-6,11-
dihydro-6-methyl-11-[(8-methyl-6-azabicyclo[3.2.1]oct-3-yl)oxy]dibenzo[c,f]
[1,2]thiazepine 5,5-dioxide and 6,11-dihydro-6-methyl-11-(8-methyl-8-
azabicyclo[3.2.1]octan-3α-yloxy)dibenzo[cf][1,2]thiazepin-5,5-dioxide).
The above chloro intermediate product, 3 g and 3.5 g of tropine with 25 ml of
toluene as reaction medium are refluxed for 3 hrs. The reaction mixture is
then concentrated in a vacuum until the solvent and other volatile materials
are removed. The residue is treated with dilute aqueous hydrochloric acid, and
ether. The aqueous layer is separated and neutralized with aqueous sodium
hydroxide. Insoluble material, which thereupon separates is dissolved in ether,
and the ether solution separated. It is washed several times with water, dried
and evaporated, leaving the free base form of the desired product (zepastine)
which crystallizes on standing, MP: 157°C. It is recrystallized from ethyl
acetate, MP: 162°C.
The base is converted to the hydrogen maleate salt by treatment in ethyl
acetate solution with one molecular proportion of maleic acid. This material is
recrystallized from ethanol, MP: 215°C.
Therapeutic Function
Antihistaminicá Anticholinergic
Safety information for Zepastine
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
