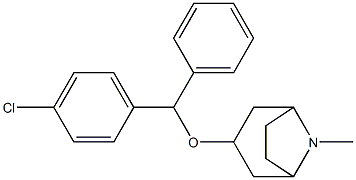
benzatropine
- CAS NO.:86-13-5
- Empirical Formula: C21H25NO
- Molecular Weight: 307.4293
- MDL number: MFCD00866568
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 22:16:43
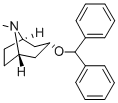
What is benzatropine?
Absorption
Oral administration of 1.5 mg of benztropine is slowly absorbed in the gastrointestinal tract and it reaches a peak concentration of 2.5 ng/ml in about 7 hours. It has an approximate oral bioavailability of 29%.
Toxicity
The oral LD50 of benztropine is reported to be of 940 mg/kg in rats. In the presence of overdose with benztropine, it has been observed symptoms of circulatory collapse, cardiac arrest, respiratory depression, respiratory arrest, psychosis, shock, coma, seizure, ataxia, combativeness, anhidrosis, hyperthermia, fever, dysphagia, decreased bowel sounds and sluggish pupils.
The Uses of benzatropine
Antiparkinsonian.
Background
Benztropine, with the chemical formula 3alpha-diphenylmethoxytropane, is a tropane-based dopamine inhibitor used for the symptomatic treatment of Parkinson's disease. It is a combination molecule between a tropane ring, similar to cocaine, and a diphenyl ether from the dialkylpiperazines determined to be a dopamine uptake inhibitor since 1970. The generation of structure-activity relationships proved that benztropine derivatives with the presence of a chlorine substituent in the para position in one of the phenyl rings produces an increased potency for dopamine uptake inhibition as well as a decreased inhibition of serotonin and norepinephrine. Benztropine was developed by USL Pharma and officially approved by the FDA on 1996.
Indications
Benztropine is indicated to be used as an adjunct in the therapy of all forms of parkinsonism. It can also be used for the control of extrapyramidal disorders due to neuroleptic drugs.
The extrapyramidal symptoms are defined as drug-induced disorders that include symptoms of dystonia, akathisia, parkinsonism, bradykinesia, tremors, and dyskinesia.
Parkinsonism is a general term that refers to the group of neurological disorders that produce symptoms similar to Parkinson's disease such as tremors, slow movement, and stiffness. The parkinsonism includes a large number of disorders and some of them have not been clearly defined.
Definition
ChEBI: Tropane in which a hydrogen at position 3 is substituted by a diphenylmethoxy group (endo-isomer). An acetylcholine receptor antagonist, it is used (particularly as its methanesulphonate salt) in the treatment of Parkinson's disease, and to re uce parkinsonism and akathisia side effects of antipsychotic treatments.
brand name
Cogentin (Merck).
Pharmacokinetics
The inhibition of dopamine reuptake by benztropine produces a dose-dependent increase of dopamine in the nerve terminal of the dopaminergic system.
Clinically the activity of benztropine is observed after 1-2 hours of oral administration and after a few minutes of intramuscular administration with a last-longing effect of about 24 hours. Reports have indicated that benztropine has a very large sedative effect.
The antihistaminic effect of benztropine is very similar to the effect found in pyrilamine and the anticholinergic activity was found to be equal to atropine ex vivo and of about 50% activity in vivo.
Safety Profile
Poison by subcutaneous andintravenous routes. When heated to decomposition itemits toxic fumes of NOx.
Metabolism
Benztropine has been shown to undergo metabolism mainly marked by N-oxidation, N-dealkylation and ring hydroxylation. The extensive metabolism of benztropine produces eight phase-I metabolites plus four glucuronide conjugates.
Properties of benzatropine
| Boiling point: | 447.93°C (rough estimate) |
| Density | 1.0505 (rough estimate) |
| refractive index | 1.5614 (estimate) |
| pka | 10.54±0.40(Predicted) |
| EPA Substance Registry System | 8-Azabicyclo[3.2.1]octane, 3-(diphenylmethoxy)-8-methyl-, (3-endo)- (86-13-5) |
Safety information for benzatropine
Computed Descriptors for benzatropine
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
