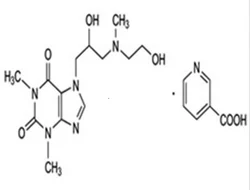
Xanthinol nicotinate
- CAS NO.:437-74-1
- Empirical Formula: C19H26N6O6
- Molecular Weight: 434.45
- MDL number: MFCD00058342
- EINECS: 207-115-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 17:32:32
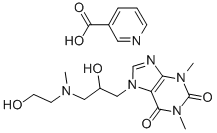
What is Xanthinol nicotinate?
The Uses of Xanthinol nicotinate
Xanthinol Nicotinate is a therapeutic agent that acts as a vasodilator.
The Uses of Xanthinol nicotinate
Sedative, Hypnotic
What are the applications of Application
Xanthinol niacinate is a useful vasodilator agent
Manufacturing Process
To a well-stirred solution of 740 parts by weight of epichlorohydrin in 200
parts by volume of isopropyl alcohol are added 600 parts by weight of
methylaminoethanol during about 3 hours at 15°C to 20°C. The heat
generated by the condensation is removed by means of a cooling bath. After
the addition of the total quantity of methylaminoethanol, stirring is continued
for 1 hour at 25°C. The condensation reaction is completed when
development of heat reaction can no longer be observed. The solution thus
produced of the raw 1-chloro-3-(methylhydroxyethylamino)-propanol-2 in
isopropyl alcohol is a colorless viscous liquid which is used without further
purification for the subsequent condensation with theophylline.
320 parts by weight of caustic soda are dissolved in 200 parts by weight of
water and diluted with 6,000 parts by weight of isopropyl alcohol. 1,584 parts
by weight of theophylline-hydrate are added to the well-stirred alcoholic
caustic soda solution having a temperature between 50°C to 60°C. As a
result, most of the theophylline sodium salt is precipitated and a doughy or
pasty white reaction product is formed. While being stirred and heated to the
boiling point of alcohol, the solution of the afore-described 1-chloro-3-
(methylhydroxyethylamino)-propanol-2 is added dropwise into the reaction
vessel during about 3 hours. After further cooking for 2 hours, the alcoholic
solution of deposited sodium chloride is filtered off. By vaporizing the alcohol,
the 3-(methylhydroxyethylamino)-2-hydroxypropyltheophylline can be
obtained as a very viscous oil which contains impurities in the form of by-
products.
For purpose of purification, the hot alcoholic solution is mixed with 975 parts
by weight of nicotinic acid while being stirred and heated until the nicotinic
acid is completely dissolved.
The 3-(methylhydroxyethylamino)-2-hydroxypropyltheophylline-nicotinate separates, while still being warm, in the form of shiny, thin, small sheets.
After cooling, the crystallization product is sucked off from the mother liquor
and recrystallized from 85% isopropyl alcohol.
The melting point of the pure nicotinic acid salt is 180°C and the yield is 75%
to 80% related to the used theophylline. The substance has a nearly neutral
reaction and is very readily soluble in water.
Therapeutic Function
Vasodilator
Properties of Xanthinol nicotinate
| Melting point: | 180° |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Water (Slightly) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Water: 250 mg/mL (575.44 mM) |
| CAS DataBase Reference | 437-74-1(CAS DataBase Reference) |
Safety information for Xanthinol nicotinate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Xanthinol nicotinate
Xanthinol nicotinate manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Xanthinol niacinate 99%View Details
Xanthinol niacinate 99%View Details
437-74-1 -
 Xanthinol niacinate 437-74-1 99%View Details
Xanthinol niacinate 437-74-1 99%View Details
437-74-1 -
 Xanthinol nicotinate 95% CAS 437-74-1View Details
Xanthinol nicotinate 95% CAS 437-74-1View Details
437-74-1 -
 Xanthinol NicotinateView Details
Xanthinol NicotinateView Details
437-74-1 -
 Xanthinol Nicotinate APIView Details
Xanthinol Nicotinate APIView Details
437-74-1 -
 XANTHINOL NICOTINATE apiView Details
XANTHINOL NICOTINATE apiView Details
437-74-1 -
 Xanthinol Nicotinate CAS 437-74-1View Details
Xanthinol Nicotinate CAS 437-74-1View Details
437-74-1 -
 Xanthinol Nicotinate Pharma APIView Details
Xanthinol Nicotinate Pharma APIView Details
437-74-1
