1,1,1,2-Tetrafluoroethane
Synonym(s):HFC-134a
- CAS NO.:811-97-2
- Empirical Formula: C2H2F4
- Molecular Weight: 102.03
- MDL number: MFCD00066608
- EINECS: 212-377-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:08
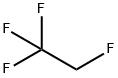
What is 1,1,1,2-Tetrafluoroethane?
Chemical properties
colourless gas or cryogenic liquid with an ether-like odour
Chemical properties
Tetrafluoroethane is a liquefied gas and exists as a liquid at room temperature when contained under its own vapor pressure, or as a gas when exposed to room temperature and atmospheric pressure. The liquid is practically odorless and colorless. The gas in high concentrations has a slight etherlike odor. Tetrafluoroethane is noncorrosive, nonirritating, and nonflammable.
The Uses of 1,1,1,2-Tetrafluoroethane
Refrigerant, propellant for pharmaceuticals; blowing agent for foams.
Background
Norflurane is under investigation in clinical trial NCT01673061 (Vapocoolant Spray for Numbing Small Boils Before Incision and Drainage).
Production Methods
Tetrafluoroethane can be prepared by several different routes;
however, the following routes of preparation illustrate the methods
used:
Isomerization/hydrofluorination of 1,1,2-trichloro-1,2,2-trifluoroethane
(CFC-113) to 1,1-dichloro-1,2,2,2-tetrafluoroethane
(CFC-114a), followed by hydrodechlorination of the latter.
Hydrofluorination of trichloroethylene, via 1-chloro-1,1,1-
trifluoroethane (HCFC-133a).
General Description
A colorless gas with a slight ethereal odor. Vapors are heavier than air. Shipped liquefied under own vapor pressure. Flash point 351°F. Inhalation at high concentrations is harmful and may cause heart irregularities, unconsciousness or death without warning. Liquid contact may cause frostbite. Vapors can replace the available oxygen.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1,1,1,2-Tetrafluoroethane is chemically inert in many situations, but can react violently with strong reducing agents such as the very active metals and the active metals. Can react with strong oxidizing agents or weaker oxidizing agents under extremes of temperature.
Health Hazard
Vapors may cause dizziness or asphyxiation without warning. Vapors from liquefied gas are initially heavier than air and spread along ground. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating, corrosive and/or toxic gases.
Fire Hazard
Some may burn but none ignite readily. Containers may explode when heated. Ruptured cylinders may rocket.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Tetrafluoroethane is a hydrofluorocarbon (HFC) or hydrofluoroalkane
(HFA) aerosol propellant (contains hydrogen, fluorine, and
carbon) as contrasted to a CFC (chlorine, fluorine, and carbon). The
lack of chlorine in the molecule and the presence of hydrogen reduce
the ozone depletion activity to practically zero. Hence tetrafluoroethane
is an alternative to CFCs in the formulation of metereddose
inhalers (MDIs). It has replaced CFC-12 as a refrigerant
and propellant since it has essentially the same vapor pressure. Its
very low Kauri-butanol value and solubility parameter indicate that
it is not a good solvent for the commonly used surfactants for MDIs.
Sorbitan trioleate, sorbitan sesquioleate, oleic acid, and soya
lecithin show limited solubility in tetrafluoroethane and the amount
of surfactant that actually dissolves may not be sufficient to keep a
drug readily dispersed. Up to 10% ethanol may be used to increase
its solubility.
When tetrafluoroethane (P-134a) is used for pharmaceutical
aerosols and MDIs, the pharmaceutical grade must be specified.
Industrial grades may not be satisfactory due to their impurity
profiles.
Safety
Tetrafluoroethane is used as a refrigerant and as a non-CFC propellant in various aerosols including topical pharmaceuticals and MDIs. Tetrafluoroethane is regarded as nontoxic and nonirritating when used as directed. No acute or chronic hazard is present when exposures to the vapor are below the acceptable exposure limit (AEL) of 1000 ppm, 8-hour and 12-hour time weighed average (TWA). In this regard it has the same value as the threshold limit value (TLV) for CFC-12. Inhaling a high concentration of tetrafluoroethane vapors can be harmful and is similar to inhaling vapors of CFC-12. Intentional inhalation of vapors of tetrafluoroethane can be dangerous and may cause death. The same labeling required on CFC aerosols would be required for those containing tetrafluoroethane as a propellant (except for the EPA requirement).
Carcinogenicity
The results from three lifetime
inhalation carcinogenesis studies with HFC 134a have been
published. The first one involved exposure of groups of
80 male and 80 female rats to levels of ≤50,000 ppm 6 h/
day, 5 days/week for 2 years.An increase inLeydig cell tumors
was seen in themale rats at 50,000 ppm(30%) compared to the
air-exposed controls (12%). Likewise, therewas an increase in
Leydig cell hyperplasia. No effects were seen at 10,000 ppm
(370). The second study with rats involved snout-only inhalation
exposures to levels of ≤50,000 ppm 1 h/day, 7 days/week
for 108 weeks. The same investigators conducted a lifetime
study withmice. In this study, groups of mice were exposed to
snout-only levels of ≤75,000 ppm 1 h/day, 7 days/week for
104 weeks. No adverse effects were seen in either rats or
mice. Since the total dose received by the rats in the high
exposure level of this study was lower than in the Collins’
study, this report supports the observation that 10,000 ppm,
6 h/day, 5 days/week for 2 years was a NOEL.
Rats were given 300 mg of HFC 134a in corn oil 5 days/
week for 52 weeks and held for a total of 125 weeks.
There was no evidence for carcinogenicity.
Metabolism
Not Available
Storage
Tetrafluoroethane is a nonreactive and stable material. The liquified gas is stable when used as a propellant and should be stored in a metal cylinder in a cool dry place.
Incompatibilities
The major incompatibility of tetrafluoroethane is its lack of miscibility with water. Since it has a very low Kauri-butanol value, tetrafluoroethane is considered to be a very poor solvent for most drugs used in MDI formulations. It also shows a low solubility for some of the commonly used MDI surfactants.
Regulatory Status
Included in the FDA Inactive Ingredients Database (aerosol formulations for inhalation and nasal applications). Included in nonparenteral medicines licensed in the UK.
Properties of 1,1,1,2-Tetrafluoroethane
| Melting point: | -101°C |
| Boiling point: | −26.5 °C(lit.) |
| Density | 1.21 |
| refractive index | 1.0007 |
| solubility | Soluble in ethanol (95%), ether, and 1 in 1294 parts of
water at 20℃. |
| Water Solubility | 1g/L at 25℃ |
| Merck | 13,4734 |
| Stability: | Stable. May cause damage to the atmosphere. Incompatible with active metals, strong oxidizing agents. |
| CAS DataBase Reference | 811-97-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethane, 1,1,1,2-tetrafluoro-(811-97-2) |
| EPA Substance Registry System | HFC-134a (811-97-2) |
Safety information for 1,1,1,2-Tetrafluoroethane
| Signal word | Warning |
| Pictogram(s) |
 Gas Cylinder Compressed Gases GHS04 |
| GHS Hazard Statements |
H280:Gases under pressure |
| Precautionary Statement Codes |
P410+P403:Protect from sunlight. Store in a well-ventilated place. |
Computed Descriptors for 1,1,1,2-Tetrafluoroethane
| InChIKey | LVGUZGTVOIAKKC-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

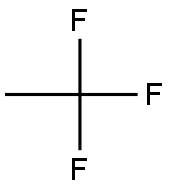


You may like
-
 KIRO Refrigerant Gas R134a Cylinder, 10 kg, 60 kgView Details
KIRO Refrigerant Gas R134a Cylinder, 10 kg, 60 kgView Details
811-97-2 -
 HT-650 Aastu Combine Deep Freezer, Refrigerant Used: R134aView Details
HT-650 Aastu Combine Deep Freezer, Refrigerant Used: R134aView Details
811-97-2 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
