Tezacaftor
- CAS NO.:1152311-62-0
- Empirical Formula: C26H27F3N2O6
- Molecular Weight: 520.5
- MDL number: MFCD23106064
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
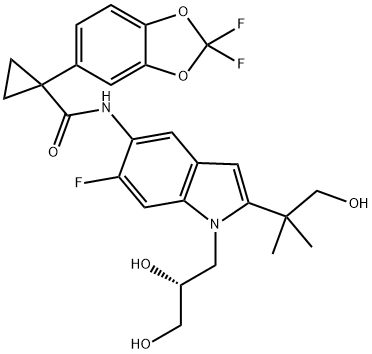
What is Tezacaftor?
Absorption
The Cmax, Tmax and AUC of tezacaftor, when administered with ivacaftor, are 5.95 mcg/ml, 2-6 h, and 84.5 mcg.h/ml respectively. Exposure of tezacaftor/ivacaftor increases 3-fold when it is administered with a high-fat meal.
Toxicity
The LD50 of an oral dose in rats is >2000 mg/kg.
Overdose symptoms may include dizziness and diarrhea. There have been no reports to this date of tezacaftor overdose, but the highest dose of 450 mg every 12 hours commonly resulted in reports of dizziness and diarrhea. No antidote exists for treating an overdose with this drug. General supportive measures should be undertaken along with monitoring of vital signs and close monitoring of clinical status.
Description
Tezacaftor (VX-661) is an oral medication for cystic fibrosis (CF) developed by Vertex Pharmaceuticals. In a combination therapy formulation called Symdeko (tezacaftor plus ivacaftor), it is approved in the U.S. and Canada for CF patients, ages 12 and older, who have two copies of the F508del mutation in the CFTR gene and one minimal function mutation. Symdeko is approved and marketed in the EU as Symkevi.Tezacaftor is not approved as a stand-alone treatment, but as part of a combination therapy.
The Uses of Tezacaftor
Tezacaftor is used as a combination therapy with Ivacaftor for the treatment of patients with cystic fibrosis.
Background
Tezacaftor is a drug of the cystic fibrosis transmembrane conductance regulator (CFTR) potentiator class. It was developed by Vertex Pharmaceuticals and FDA approved in combination with ivacaftor to manage cystic fibrosis. This drug was approved by the FDA on February 12, 2018.
Cystic Fibrosis is an autosomal recessive disorder caused by one of several different mutations in the gene for the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) protein, an ion channel involved in the transport of chloride and sodium ions across cell membranes. CFTR is active in epithelial cells of organs such as of the lungs, pancreas, liver, digestive system, and reproductive tract. Alterations in the CFTR gene result in altered production, misfolding, or function of the protein and consequently abnormal fluid and ion transport across cell membranes. As a result, CF patients produce thick, sticky mucus that clogs the ducts of organs where it is produced making patients more susceptible to complications such as infections, lung damage, pancreatic insufficiency, and malnutrition.
Indications
Tezacaftor is combined with ivacaftor in one product for the treatment of cystic fibrosis (CF) in patients aged 12 years or older with two copies of the F508del gene mutation or at least one mutation in the CFTR gene that is responsive to this drug.
Tezacaftor, when used in combination with ivacaftor and elexacaftor in the product Trikafta, is also indicated for the treatment of CF in patients 12 years of age and older that have at least one F508del mutation in the CFTR gene.
Definition
VX-661 is an investigational compound that promotes the maturation of delta F508 mutants of the cystic fibrosis transmembrane conductance regulator (CFTR). Delta F508 CFTR represents a class of CFTR mutation that is characterized by impaired processing of misfolded CFTR proteins and reduced accumulation of the protein at the cell surface. VX-661 is intended to facilitate trafficking of CFTR to the epithelial cell membrane. It may be combined with the CFTR potentiator ivacaftor (Item No. 15145) to stimulate both CFTR accumulation and opening at the apical epithelial surface.
Biochem/physiol Actions
VX-661 is another cystic fibrosis transmembrane conductance regulator (CFTR) corrector in development for the treatment of cystic fibrosis.
Pharmacokinetics
Clinical studies have shown a significant decrease in sweat chloride and an increase in the forced expiratory volume (FEV), a measure of lung function, following Tevacaftor/Ivacaftor therapy. Phase 3 clinical studies have shown that a significant increase in forced expiratory volume was attained at 4 and 8 weeks after initiating this drug. The above effects lead to improvement of the respiratory symptoms of cystic fibrosis. Tezacaftor does not induce clinically significant QT prolongation. When given with ivacaftor, tezacaftor can lead to liver transaminase elevations. Testing of transaminases (ALT and AST) levels should occur before starting this combination every 3 months during the first year of treatment, and every year afterwards. Patients with a history of transaminase elevations should be monitored more frequently.
in vitro
VX-661, is CFTR modulator that is potentially useful for treatment of cystic fibrosis. VX-661 corrects F508del-CFTR trafficking and increases F508del-CFTR protein activity in vitro.
Metabolism
Tezacaftor is metabolized extensively in humans by the action of CYP3A4 and CYP3A5. There are three main circulating metabolites; M1, M2, and M5. The M1 is an active metabolite with similar activity to the parent drug, tezacaftor. The M2 metabolite is significantly less active and M5 is considered an inactive metabolite. An additional circulating metabolite, M3, corresponding to the glucuronide form of tezacaftor.
References
[1] s. donaldson, j. pilewski, m. griese, q. dong, p.-s. lee, for the vx11–661-101 study group. ws7.3 vx-661, an investigational cftr corrector, in combination with ivacaftor, a cftr potentiator, in patients with cf and homozygous for the f508del-cftr mutation: interim analysis. journal of cystic fibrosis, volume 12, supplement 1, june 2013, page s14
Properties of Tezacaftor
| Boiling point: | 610.8±55.0 °C(Predicted) |
| Density | 1.49±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C , stored under nitrogen |
| solubility | ≥21.8 mg/mL in DMSO; insoluble in EtOH; ≥24.3 mg/mL in H2O |
| form | solid |
| pka | 13.99±0.20(Predicted) |
| color | White to yellow |
Safety information for Tezacaftor
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Tezacaftor
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1H-Pyrrole-2-carboxaMide, 4-[2-[(2-chloro-4-fluorophenyl)aMino]-5-Methyl-4-pyriMidinyl]-N-[(1S)-1-(3-chlorophenyl)-2-hydroxyethyl]-](https://img.chemicalbook.in/CAS/GIF/896720-20-0.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1