Ataluren
Synonym(s):3-(5-(2-Fluorophenyl)-1,2,4-oxadiazol-3-yl)benzoic acid, Premature Termination Codon Mutations Inhibitor, PTC124, Ataluren;PTC-124 - CAS 775304-57-9 - Calbiochem
- CAS NO.:775304-57-9
- Empirical Formula: C15H9FN2O3
- Molecular Weight: 284.24
- MDL number: MFCD09864996
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
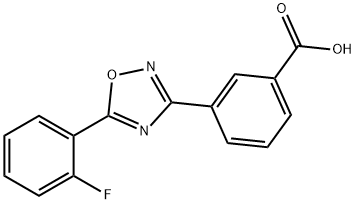
What is Ataluren?
Absorption
Peak plasma levels of ataluren are attained approximately 1.5 hours after dosing in subjects who received medicinal product within 30 minutes of a meal .
Description
Ataluren is a drug marketed under the trade name Translarna® which was developed by PTC Therapeutics and approved by the European Union in May 2014 for the treatment of Duchenne’s muscular dystrophy (DMD) and potentially other genetic disorders. Ataluren renders ribosomes less sensitive to premature stop or ‘read-through’ codons, which are thought to be beneficial in diseases such as DMD and cystic fibrosis.
Description
Nonsense mutations create a premature termination of mRNA translation and have been implicated in various genetic disorders, including muscular dystrophy and cystic fibrosis. PTC-124 is a nonaminoglycoside that has been reported to selectively induce ribosomes to read through premature nonsense stop signals on mRNA, thus allowing the production of full length, functional proteins. In a mouse model of cystic fibrosis caused by nonsense mutations, PTC-124 treatment (60 mg/kg s.c. injection or 0.3-0.9 mg/ml orally) has been shown to restore cystic fibrosis transmembrane conductance regulator (CFTR) protein expression and function. The target activity of PTC-124 was initially evaluated by firefly luciferase reporter cell-based nonsense codon assay (IC50 = 7 nM); however, subsequent assessments using a Renilla reniformis luciferase reporter have failed to produce nonsense codon suppression activity. Thus, while PTC-124 is in clinical testing in patients with nonsense mutations within the CFTR or dystrophin genes, controversy surrounds its exact mechanism of action.
The Uses of Ataluren
Nonsense mutations create a premature termination of mRNA translation and have been implicated in various genetic disorders, including muscular dystrophy and cystic fibrosis. PTC-124 is a nonaminoglycoside that has been reported to selectively induce ribosomes to read through premature nonsense stop signals on mRNA, thus allowing the production of full-length, functional proteins. In a mouse model of cystic fibrosis caused by nonsense mutations, PTC-124 treatment (60 mg/kg s.c. injection or 0.3-0.9 mg/ml orally) has been shown to restore cystic fibrosis transmembrane conductance regulator (CFTR) protein expression and function. The target activity of PTC-124 was initially evaluated by firefly luciferase reporter cell-based nonsense codon assay (IC50 = 7 nM); however, subsequent assessments using a Renilla reniformis luciferase reporter have failed to produce nonsense codon suppression activity. Thus, while PTC-124 is in clinical testing in patients with nonsense mutations within the CFTR or dystrophin genes, controversy surrounds its exact mechanism of action.[Cayman Chemical]
The Uses of Ataluren
PTC-124 is a nonaminoglycoside that has been reported to induce ribosomes to read through premature nonsense stop signals on mRNA, allowing the production of full-length functional proteins,
Background
Ataluren is a novel, orally administered drug that targets nonsense mutations. Ataluren is approved for use by the European Medicines Agency to treat Duchenne Muscular Dystrophy in patients aged 5 years and older who are able to walk. More specifically, ataluren is used in the small group of patients whose disease is caused by a specific genetic defect (called a ‘nonsense mutation’) in the dystrophin gene.
This drug does not yet have approval by the US Food and Drug Administration or by Health Canada for any indications.
What are the applications of Application
PTC124 is a firefly luciferase inhibitor and an inducer of ribosomal read-through of mRNAs
Indications
Ataluren is approved for use by the European Medicines Agency to treat Duchenne Muscular Dystrophy in patients aged 5 years and older who are able to walk. More specifically, ataluren is used in the small group of patients whose disease is caused by a specific genetic defect (called a ‘nonsense mutation’) in the dystrophin gene.
Definition
ChEBI: 3-[5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl]benzoic acid is a ring assembly and an oxadiazole.
Synthesis
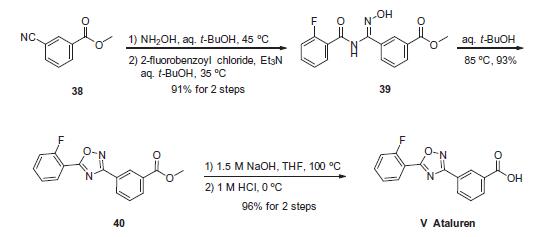
The sequence to construct ataluren, which was described by the authors at PTC Therapeutics, commenced with commercially available methyl 3-cyanobenzoate (38). This ester was exposed to hydroxylamine in aqueous tert-butanol and warmed gently until the reaction was deemed complete. Then this mixture was treated with 2-fluorobenzoyl chloride dropwise and subsequently triethylamine dropwise. To minimize exotherm and undesired side products, careful control of the addition of reagents was achieved through slow dropwise addition of these liquid reagents. Upon complete consumption of starting materials and formation of amidooxime 39, the aqueous reaction mixture was then heated to 85 ?? to facilitate 1,2,4-oxadiazole formation, resulting in the tricyclic ester 40 in excellent yield across the three steps. Finally, saponification of ester 40 through the use of sodium hydroxide followed by acidic quench gave ataluren (V) in 96% over the two-step sequence.
Metabolism
Ataluren is metabolized by conjugation via uridine diphosphate glucuronosyltransferase (UGT) enzymes, predominantly UGT1A9 in liver and intestine. In vivo, the only metabolite detected in plasma after oral administration of radio-labelled ataluren was the ataluren-O-1β-acyl glucuronide; exposure to this metabolite in humans was approximately 8% of the plasma AUC of ataluren .
Properties of Ataluren
| Melting point: | 241 - 242°C |
| Boiling point: | 503.7±60.0 °C(Predicted) |
| Density | 1.379 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly) |
| form | White to off-white solid. |
| pka | 3.58±0.10(Predicted) |
| color | White to Off-White |
| InChI | InChI=1S/C15H9FN2O3/c16-12-7-2-1-6-11(12)14-17-13(18-21-14)9-4-3-5-10(8-9)15(19)20/h1-8H,(H,19,20) |
| CAS DataBase Reference | 775304-57-9 |
Safety information for Ataluren
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H336:Specific target organ toxicity,single exposure; Narcotic effects H373:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P314:Get medical advice/attention if you feel unwell. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for Ataluren
| InChIKey | OOUGLTULBSNHNF-UHFFFAOYSA-N |
| SMILES | C(O)(=O)C1=CC=CC(C2N=C(C3=CC=CC=C3F)ON=2)=C1 |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![1-[[(2S,3S)-3-(2-Chlorophenyl)-2-(4-fluorophenyl)oxiran-2-yl]methyl]-1,2,4-triazole](https://img.chemicalbook.in/CAS/GIF/133855-98-8.gif)
You may like
-
 Ataluren 98% CAS 775304-57-9View Details
Ataluren 98% CAS 775304-57-9View Details
775304-57-9 -
 PTC-124 CAS 775304-57-9View Details
PTC-124 CAS 775304-57-9View Details
775304-57-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1