Vorapaxar
- CAS NO.:618385-01-6
- Empirical Formula: C29H33FN2O4
- Molecular Weight: 492.58
- MDL number: MFCD16038876
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
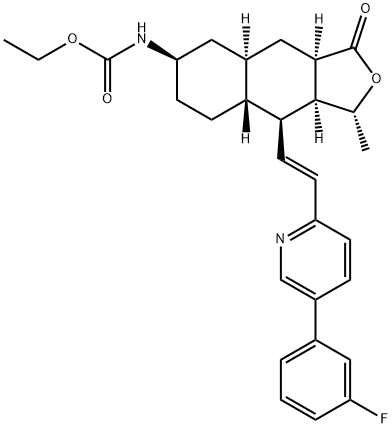
What is Vorapaxar?
Absorption
After oral administration, vorapaxar is rapidly absorbed and peak concentrations occur at a median tmax of 1 hour under faster conditions. Vorapaxar may be taken with or without food as ingestion with a high-fat meal did not result in meaningful changes in AUC. The mean absolute bioavailability is 100%.
Toxicity
There is an increased risk of bleeding and intracranial hemorrhage (ICH), which is why the use of vorapaxar is contraindicated in patients with a history of stroke, trans-ischemic attack (TIA), ICH, or active pathological bleeding such as peptic ulcer. Animal studies have suggested that there is a low probability of embryo/fetal toxicities, however there are no adequate and well-controlled studies describing use in pregnant women. Vorapaxar should also be avoided during breastfeeding as it is unknown whether vorapaxar or its metabolites are excreted in human milk, however it has been shown to be actively secreted in the milk of rats.
Description
Vorapaxar is an orally bioavailable competitive antagonist of the proteinase-activated receptor (PAR1; Ki = 8.1 nM), also known as the thrombin receptor. It is selective for PAR1 over other PARs, as well as a number of GPCRs, ion channels, and receptors. It inhibits platelet aggregation induced by thrombin and haTRAP (IC50s = 47 and 25 nM, respectively). Vorapaxar (0.1 mg/kg, i.v.) completely inhibits platelet aggregation in cynomolgus monkeys ex vivo. Formulations containing vorapaxar are used in the prevention of thrombotic cardiovascular events.
Indications
Vorapaxar is indicated for the reduction of thrombotic cardiovascular events in patients with a history of myocardial infarction (MI) or peripheral arterial disease (PAD). It is usually co-administered with acetylsalicylic acid (ASA) and/or clopidogrel, and should therefore be administered as an addition to these medications as it has not been studied alone.
Background
Vorapaxar is a tricyclic himbacine-derived selective inhibitor of protease activated receptor (PAR-1) indicated for reducing the incidence of thrombotic cardiovascular events in patients with a history of myocardial infarction (MI) or with peripheral arterial disease (PAD). By inhibiting PAR-1, a thrombin receptor expressed on platelets, vorapaxar prevents thrombin-related platelet aggregation.
Definition
ChEBI: A carbamate ester that is the ethyl ester of [(1R,3aR,4aR,6R,8aR,9S,9aS)-9-{(E)-2-[5-(3-fluorophenyl)pyridin-2-yl]ethyny }-1-methyl-3-oxododecahydronaphtho[2,3-c]furan-6-yl]carbamic acid. A protease-activated receptor-1 antagonist used (as its sulfate salt) for the reduction of thrombotic cardiovascular events in patients with a history of myocardial infarction MI) or with peripheral arterial disease. It has been shown to reduce the rate of a combined endpoint of cardiovascular death, MI, stroke and urgent coronary revascularisation.
Metabolism
Vorapaxar is metabolized to its major circulating metabolite, M20, and its predominant metabolite excreted into feces, M19, by CYP3A4 and CYP 2J2.
References
[1]. chackalamannil s & xia, y. thrombin receptor (par-1) antagonists as novel antithrombotic agents. expert opinion on therapeutic patents, 2006.16:493-505.
[2]. chackalamannil s, wang y, greenlee w j et al. 2008. discovery of a novel, orally active himbacine-based thrombin receptor antagonist (sch 530348) with potent antiplatelet activity. j med chem, 2008,51: 3061-3064.
Properties of Vorapaxar
| Boiling point: | 676.0±55.0 °C(Predicted) |
| Density | 1.24±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20° C |
| solubility | ≥24.65 mg/mL in DMSO; insoluble in H2O; ≥10.64 mg/mL in EtOH with ultrasonic |
| form | solid |
| pka | 12.49±0.60(Predicted) |
| color | White to off-white |
Safety information for Vorapaxar
Computed Descriptors for Vorapaxar
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![(1R,3aR,4aR,8aR,9S,9aR)-1-methyl-3-oxodecahydro-3H-spiro[naphtho[2,3-c]furan-6,2'-[1,3]dioxolane]-9-carboxylic acid](https://img.chemicalbook.in/CAS/20150408/GIF/900161-13-9.gif)
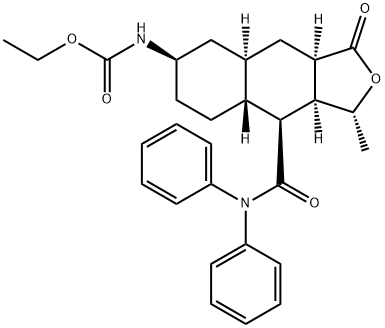
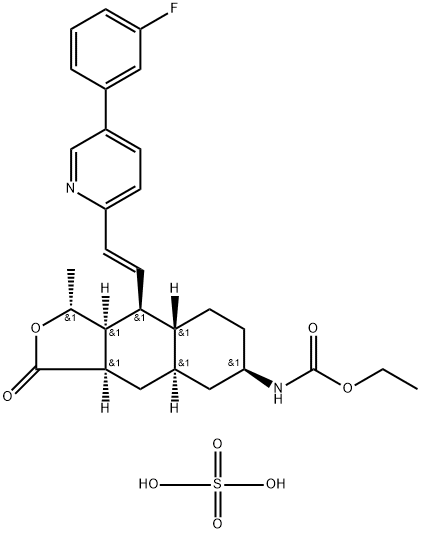
![(3R,3aS,4S,4aS,7R,9aR)-3-Methyl-7-nitro-1-oxo-N,N-diphenyl-1,3,3a,4,4a,5,6,7,8,9a-decahydronaphtho[2,3-c]furan-4-carboxamide](https://img.chemicalbook.in/CAS/20180703/GIF/900186-72-3.gif)

You may like
-
 Vorapaxar 98% (HPLC) CAS 618385-01-6View Details
Vorapaxar 98% (HPLC) CAS 618385-01-6View Details
618385-01-6 -
 Vorapaxar CAS 618385-01-6View Details
Vorapaxar CAS 618385-01-6View Details
618385-01-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8