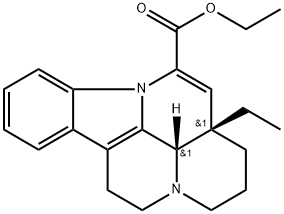
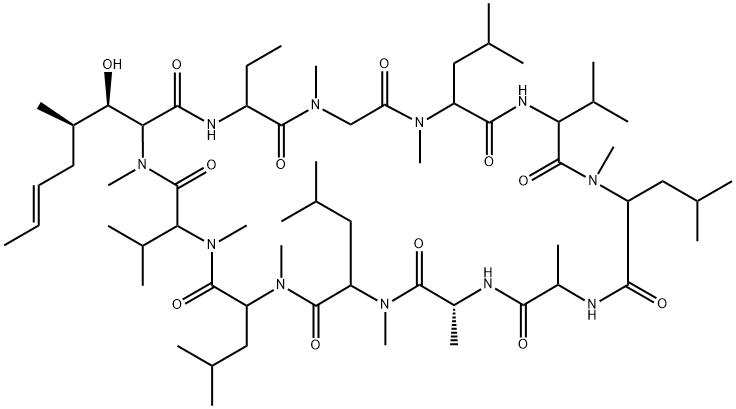
Vincristine
- CAS NO.:57-22-7
- Empirical Formula: C46H56N4O10
- Molecular Weight: 824.96
- MDL number: MFCD16621723
- EINECS: 200-318-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57

What is Vincristine?
Toxicity
IVN-RAT LD50 1300 mg/kg; IPR-MUS LD50 5.2 mg/kg. Marqibo? must only be administered IV because it is fatal if administered by other routes. Marqibo? also has different dosing than vincristine sulphate injection, so attention is needed to prevent overdoses. The most clinically significant adverse effect of vincristine is neurotoxicity.
Description
A complex dimeric alkaloid isolated from Vinca minor, this base has the structure given above based upon chemical and spectroscopic data. It resembles Vinblastine in its pharmacological action. Depending on the dosage, it causes either thrombocytosis with no effect upon the leucocytes, or thrombocytopenia accompanied by peucopenia in rats. Thrombocytosis persists even after prolonged treatment with drugs given in the correct dosage.
Chemical properties
solid
Physical properties
Appearance: needlelike crystals were produced when recrystallized by methanol. The other properties of vincristine are similar to vinblastine.
Originator
Oncovin,Lilly ,US ,1963
History
Vinblastine and vincristine were extracted from vinca, and researchers found that they are the main ingredient of vinca which have antitumor activity. This is an accidental harvest by hard work and inspiration during scientific research process.
It was firstly reported as an important discovery by Annals of the New York Academy of Sciences, and one report was published by Robert Noble and Charles Thomas Beer . They demonstrated that vinblastine can cause severe leukopenia.
In 1963, vincristine (trade name, Oncovin) developed by Eli Lilly was approved by the US FDA, it provided a good choice for tumor chemotherapy program and gradually became the essential tumor chemotherapy drugs.
At the early stages of the study, vinblastine and vincristine are directly extracted from the vinca, but the cost is high, and the extraction efficiency is low . Especially the specific chiral monomer compounds with biological activity are difficult to obtain. In the following years, the researchers used genetic engineering, biosynthesis, chemical synthesis, and other technical methods to explore and optimize the production process of vinblastine drugs. In particular, the rise of asymmetric synthesis provides a more economical choice for the synthesis of vinblastine compounds and increases productivity to more than 22% . At the same time, the pharmaceutical dosage forms were developed from the beginning as simple sulfate injection to liposomal preparations and nanoparticle preparations, which improve the efficacy, reduce adverse reactions, and play better antitumor effect.
The Uses of Vincristine
Antineoplastic[Note—Graphic formula same as for Vinblastine Sulfate, except that R is CHO].
Background
Vincristine is an antitumor vinca alkaloid isolated from Vinca Rosea. It is marketed under several brand names, many of which have different formulations such as Marqibo (liposomal injection) and Vincasar. Vincristine is indicated for the treatment of acute leukaemia, malignant lymphoma, Hodgkin's disease, acute erythraemia, and acute panmyelosis. vincristine sulfate is often chosen as part of polychemotherapy because of lack of significant bone–marrow suppression (at recommended doses) and of unique clinical toxicity (neuropathy).
Indications
Treatment of acute lymphocytic leukemia (ALL), Hodgkin lymphoma, non-Hodgkin lymphomas, Wilms' tumor, neuroblastoma, rhabdomyosarcoma. Liposomal vincristine is indicated for the treatment of relapsed Philadelphia chromosome-negative (Ph-) acute lymphoblastic leukemia (ALL).
Indications
It was recorded in the Pharmacopoeia of the People’s Republic of China (2015), the
British Pharmacopoeia (2017), the United States Pharmacopeia (40), the Japanese
Pharmacopoeia (17th ed.), the European Pharmacopoeia (9th ed.), and The
International Pharmacopoeia (5th ed.).
Vincristine sulfate injection is used to treat leukemia, lymphadenoma, non-small
cell lung cancer, nephroblastoma, and neuroblastoma in clinical practice generally,
but the effect of vinblastine is better than vincristine in Hodgkin lymphoma
treatment.
Definition
ChEBI: Vincristine is a vinca alkaloid with formula C46H56N4O10 found in the Madagascar periwinkle, Catharanthus roseus. It is used (commonly as the corresponding sulfate salt)as a chemotherapy drug for the treatment of leukaemia, lymphoma, myeloma, breast cancer and head and neck cancer. It has a role as a tubulin modulator, a microtubule-destabilising agent, a plant metabolite, an antineoplastic agent and a drug. It is a methyl ester, an acetate ester, a tertiary alcohol, a member of formamides, an organic heteropentacyclic compound, an organic heterotetracyclic compound, a tertiary amino compound and a vinca alkaloid. It is a conjugate base of a vincristine(2+). It derives from a hydride of a vincaleukoblastine.
Manufacturing Process
The alkaloid mixture from the extraction of Vinca rosea plants (as in
vinblastine extraction) was chromatographed to give vincristine which was
then converted to the sulfate, according to US Patent 3,205,220.
Vincristine may also be prepared in a semisynthetic process starting from
vinblastine. Vinblastine or a salt thereof, preferably the sulfate, is oxidized
with chromic acid or with one of its salts at a low temperature, the reaction
mixture is neutralized or rendered alkaline and the product is separated
therefrom by extraction, the extract is evaporated to dryness, the dry residue
is optionally formylated, vincristine, and optionally N-demethylvinblastine also,
are isolated from the product, and the product(s) are optionally converted into
their salts; preferably into the sulfates, according to US Patent 3,899,493.
brand name
Oncovin (Lilly); Vincasar (Sicor).
Therapeutic Function
Cancer chemotherapy
General Description
A white crystalline solid. Melting point 218°C. Used as an antineoplastic.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Flash point data for Vincristine are not available, but Vincristine is probably combustible.
Pharmacokinetics
Vincristine is a vinca alkaloid antineoplastic agent used as a treatment for various cancers including breast cancer, Hodgkin's disease, Kaposi's sarcoma, and testicular cancer. The vinca alkaloids are structurally similar compounds comprised of 2 multiringed units, vindoline and catharanthine. The vinca alkaloids have become clinically useful since the discovery of their antitumour properties in 1959. Initially, extracts of the periwinkle plant (Catharanthus roseus) were investigated because of putative hypoglycemic properties, but were noted to cause marrow suppression in rats and antileukemic effects in vitro. Vincristine binds to the microtubular proteins of the mitotic spindle, leading to crystallization of the microtubule and mitotic arrest or cell death. Vincristine has some immunosuppressant effect. The vinca alkaloids are considered to be cell cycle phase-specific.
Pharmacology
Vinblastine drugs have a strong inhibitory effect on monozygotic leukemia, breast
cancer, liver cancer, ovarian cancer, head and neck cancer, testicular cancer, solid
sarcoma, and malignant melanin in a variety of spontaneous or transplanted lymphocytic leukemias. Although the structure of vinblastine and vincristine is very
similar, there are some differences in pharmacological effects, and there is no crossresistance .
The mechanism of vinblastine and vincristine is similar as cell cycle-specific
antineoplastic agents. They all inhibit tubulin polymerization, interfere spindle
microtubule formation, block the cell cycle in M phase, and then inhibit the proliferation of cancer cells . X-ray diffraction results showed that Vinblastine targets
at the hydrophobic structure on tubulin, Vinblastine inserted into the trough as the
wedge, blocking the polymerization of tubulin, making a spirochete structure of
tubulin itself form, and loss of biological role . However, this antitumor effect
can affect other rapid proliferation cells, and then small intestinal epithelial cells
and bone marrow cells will be inhibited, which is one of the reasons of gastrointestinal side effects and bone marrow transplantation side effects. The effect of vincristine is better than vinblastine, and the dosage is lower, the probability and intensity
of side effects are lower. Recently, it has been found that vinblastine and vincristine
can also suppress growth of tumor cell by inhibiting the synthesis of RNA and
protein.
Clinical Use
Vincristine is an important component of the curative combination chemotherapy for acute lymphoblastic leukemia, Hodgkin’s disease (the MOPP regimen), and non-Hodgkin’s lymphomas. It is also used in several regimens for pediatric solid tumors, including Wilms’ tumor, Ewing’s sarcoma, rhabdomyosarcoma, and neuroblastoma; in adult tumors of the breast, lung, and cervix; and in sarcomas. Its relative lack of myelosuppression makes it more attractive than vinblastine for use in combination with myelotoxic drugs.Vinblastine is especially useful in testicular carcinomas and is also active in Hodgkin’s disease, other types of lymphomas, breast cancer, and renal cell carcinoma.
Side Effects
All vinca alkaloids are severe vesicants that can induce necrosis, cellulitis, and/or thrombophlebitis. Proper needle placement before administration should be assured to eliminate the risk of extravasation. Unlike the tissue damage caused by the vesicant action of nitrogen mustards and antibiotic antineoplastics, cold exacerbates tissue destruction. If extravasation occurs, apply heat for 1 hour fours time a day for 3 to 5 days, coupled with local hyaluronidase injections. Vinca alkaloids are all Category D teratogens and are fatal if administered by the intrathecal route.
Metabolism
Hepatic. Cytochrome P450 isoenzymes of the CYP3A subfamily facilitate the metabolism of vincristine.
References
Mokry et al., Experientia, 18, 564 (1962) Pharmacology: Chandorkar.,lnd. J. Physiol. Pharmacol., 17, 105 (1973)
Properties of Vincristine
| Melting point: | 211-216 ºC |
| Boiling point: | 761.92°C (rough estimate) |
| alpha | D25 +17°; D25 +26.2° (ethylene chloride) |
| Density | 1.1539 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Store at -20°C, protect from light |
| solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. |
| form | Powder |
| pka | 5.4(at 25℃) |
| Stability: | Stable, but may be heat sensitive. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 57-22-7 |
| EPA Substance Registry System | Vincristine (57-22-7) |
Safety information for Vincristine
Computed Descriptors for Vincristine
Vincristine manufacturer
Green Vision Life Sciences Pvt Ltd.
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 57-22-7 Vincristine 98%View Details
57-22-7 Vincristine 98%View Details
57-22-7 -
 57-22-7 98%View Details
57-22-7 98%View Details
57-22-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1