Podophyllotoxin
- CAS NO.:518-28-5
- Empirical Formula: C22H22O8
- Molecular Weight: 414.41
- MDL number: MFCD00075290
- EINECS: 208-250-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
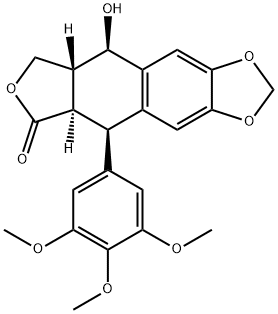
What is Podophyllotoxin?
Absorption
Topical application of 0.05 mL of 0.5% podofilox solution to external genitalia did not result in detectable serum levels. Applications of 0.1 to 1.5 mL resulted in peak serum levels of 1 to 17 ng/mL one to two hours after application.
Description
Podophyllotoxin (2,3-butyl-4-aromatic naphthene) is isolated from guijiu(Podophyllum) . There are two species as the main source of podophyllotoxin,
Podophyllum hexandrum Royle and Podophyllum peltatum .
Although podophyllotoxin has significant antitumor and antiviral activities, it
showed several toxicity and side effects. Podophyllotoxin derivatives, etoposide
(VP-16-213), Etopophos, amino sugar etoposide (NK6l1), and teniposide (VM26),
have been developed as anticancer drugs. They are used to treat small cell lung cancer, testicular cancer, acute leukemia, malignant lymphoma, etc. But podophyllotoxin derivatives are not free of toxicity. Besides the narrowing of the anticancer
spectrum and low water solubility, these drugs could induce severe myelosuppression, gastrointestinal side effects, etc.
Although the synthetic and biosynthetic pathways of podophyllotoxin have been
elucidated, it is still the most effective, economic, and fast way to extract podophyllotoxin from the plant.
Chemical properties
off-white fine crystalline powder
Physical properties
Appearance: white needle crystal powder. Solubility: freely soluble in chloroform, acetone, ethyl acetate, and benzene; soluble in ethanol and ethyl ether; and insoluble in water. Melting point: after drying the melting point is 183–184°C. Specific optical rotation: 132.7°C (chloroform).
History
Podophyllotoxin was first found in the Podophyllum peltatum L.?The first time to
isolate podophyllotoxin from podophyllin was in 1880. In 1942, it was found that
venereal warts could be effectively treated by application of podophyllin.Subsequently, podophyllotoxin was reported to inhibit the growth of the tumor
through the inhibition of the microtubule formation. The chemical structure of
podophyllotoxin was elucidated in 1951.
In the 1960s, two main podophyllotoxin derivatives were synthesized, etoposide
and teniposide (VM-26) . In 1983, etoposide was approved by FDA.?Etoposide
and teniposide are used in frontline cancer therapy against various cancer types,
such as small cell lung cancer, testicular cancer, etc. In 1996, etoposide phosphate
analog (Etopophos) was launched in America. Etopophos is the prodrug of etoposide and can be rapidly absorbed and completely converted to the parent compound
in?vivo. In 1990, WHO recommended 0.5% podophyllotoxin as the first-line drug
for the treatment of condyloma acuminatum. Podophyllotoxin creams and gels are
nowadays widely used in clinical practice.
The Uses of Podophyllotoxin
Podophyllotoxin is a non-alkaloid toxin lignan extracted from the roots and rhizomes of Podophyllum species. It binds to topoisomerase II during the late S and early G2 stage, blocking tubulin polymerization and, thus, inhibiting mitosis. In addition to being used as a cathartic, purgative, antiviral agent, vesicant, and antihelminthic, podophyllotoxin is the starting material for the semi-synthesis of the anti-cancer drugs etoposide , teniposide , and etopophos.
The Uses of Podophyllotoxin
Skin treatment for genital warts caused by some types of HPVs.
The Uses of Podophyllotoxin
antineoplastic, inhibits microtubule assembly, and human DNA topoisomerase II; antimitotic agent
Background
A lignan found in podophyllin resin from the roots of podophyllum plants. It is a potent spindle poison, toxic if taken internally, and has been used as a cathartic. It is very irritating to skin and mucous membranes, has keratolytic actions, has been used to treat warts and keratoses, and may have antineoplastic properties, as do some of its congeners and derivatives.
Indications
For treatment of external genital warts (Condyloma acuminatum).
What are the applications of Application
Podophyllotoxin is a potent inhibitor of microtubule assembly and DNA topoisomerase II
Definition
ChEBI: An organic heterotetracyclic compound that has a furonaphthodioxole skeleton bearing a 3,4,5-trimethoxyphenyl substituent. It is found in the roots and rhizomes of Podophyllum species and is used for the topical treatment of genital warts.
Indications
Podophyllotoxin (Podofilox) is available alone and as the main cytotoxic ingredient in podophyllin (25% podophyllum resin), a mixture of toxic chemicals derived from May apple plants. The active ingredients inhibit cell mitosis. The drugs are used to treat condylomata acuminata. The most common toxic effects are skin irritation and less commonly, ulceration. Systemic absorption of podophyllin can occur (especially if applied to large, inflamed areas or mucosal surfaces), with gastrointestinal, hematological, renal, and hepatotoxic effects. In addition, seizures and peripheral neuropathy have been reported.
brand name
Condylox (Oclassen).
Biochem/physiol Actions
Inhibits microtubule assembly; antineoplastic.
Pharmacokinetics
Podofilox, also called podophyllotoxin, is a purer and more stable form of podophyllin in which only the biologically active portion of the compound is present. Podofilox is used to remove certain types of warts on the outside skin of the genital areas.
Pharmacology
Antineoplastic and antiviral activities are the most pronounced pharmacological. effects of podophyllotoxin . Podophyllotoxin shows a significant inhibitory effect on the division and proliferation of epithelial cells infected by human papillomavirus (HPV), disrupts the cell cytoskeleton, and induces the necrosis and shedding of warts. It was shown that the antitumor effect of podophyllotoxin is associated with the inhibition of microtubule assembly and the induction of apoptosis. However, the antitumor effect of podophyllotoxin analogs, such as etoposide, teniposide, and Etopophos, is related to disparate mechanisms including the inhibition of DNA topoisomerase II activity and the formation of stable nucleic acid-drugenzyme complex, which induce DNA double-strand or single-strand break and eventually lead to cell death . It was also found that podophyllotoxin derivatives have immunosuppressive and anti-inflammatory effects.
Anticancer Research
Podophyllotoxin (PTOX) is an aryl-tetralin lignan and has been originallyisolated from Podophyllum peltatum L. (American podophyllum or Mayapple;family Podophyllaceae). Later, it is also isolated from several species like P.hexandrum Royle (Indian podophyllum) and P. pleianthum (Taiwanese podophyllum).PTOX has also been reported in other plants such as Linum spp., Callitrisspp., Juniperus spp., Thuja spp., Hyptis spp., Thymus spp., Teucrium spp., Nepetaspp., Dysosma spp., Diphylleia spp., and Jeffersoniana spp. (Ionkova 2007;Yousefzadi et al. 2010). PTOX shows strong cytotoxic activity against various cancercell lines. However, PTOX is too toxic for the treatment of neoplastic diseasesin humans; it is used as a precursor for chemical synthesis of semisynthetic antineoplasticdrugs, etoposide, Etopophos, and teniposide , which are successfullyused as antitumor agents (Holthuis 1988; Cragg and Newman 2005).Podophyllotoxin derivatives are used in the treatment of lymphomas, acute leukemia,and testicular, lung, ovarian, bladder, and brain cancer (Srivastava et al. 2005).Podophyllum spp. are the major source of PTOX, and their availability is limited innature, and some species are categorized as endangered. Moreover, the chemicalsynthesis of podophyllotoxin is an expensive process; therefore, biotechnologicalproduction of podophyllotoxin using plant cell and tissue cultures has been preferredby various research groups (Farkya et al. 2004).
Anticancer Research
Podophyllotoxin istoxic for humancells and is aprecursor ofsemisyntheticantineoplastic drugs(e.g., etoposide,etopophos, andteniposide).
Clinical Use
Podophyllotoxin is a useful agent for the treatment of condyloma acuminatum . Podophyllotoxin and its derivatives are also widely used in the treatment of cancer, such as lymphomas and lung carcinoma. Because of the several toxicity of podophyllotoxin, for example, the irritation of skin and mucous membranes, combination therapies are used to treat condyloma acuminatum or cancer.
Metabolism
Not Available
Purification Methods
The toxin recrystallises form *C6H6 (with 0.5C6H6), EtOH/*C6H6, aqueous EtOH (with 1-1.5H2O, m 114-115o) and CH2Cl2/pentane. When dried at 100o/10mm it has m 183-184o. [UV: Stoll et al. Helv Chim Acta 37 1747 1954, IR: Schecler et al. J Org Chem 21 288 1956.] It is an inhibitor of microtubule assembly [Prasad et al. Biochemistry 25 739 1986]. [Beilstein 19/10 V 666.]
Properties of Podophyllotoxin
| Melting point: | 183-184 °C (lit.) |
| Boiling point: | 453.31°C (rough estimate) |
| alpha | -110.7 º (c=1, CHCl3) |
| Density | 1.2649 (rough estimate) |
| refractive index | 1.4480 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO:15.0(Max Conc. mg/mL);36.2(Max Conc. mM) |
| pka | 13.26±0.40(Predicted) |
| form | Powder |
| color | White to off-white |
| optical activity | [α]/D 131±2°, c = 1 in chloroform |
| Merck | 13,7628 |
| BRN | 99163 |
| Stability: | Very Hygroscopic |
| CAS DataBase Reference | 518-28-5(CAS DataBase Reference) |
Safety information for Podophyllotoxin
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Podophyllotoxin
| InChIKey | YJGVMLPVUAXIQN-XVVDYKMHSA-N |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
You may like
-
 518-28-5 98%View Details
518-28-5 98%View Details
518-28-5 -
 Podophyllotoxin 98%View Details
Podophyllotoxin 98%View Details
518-28-5 -
 Podophyllotoxin 95.00% CAS 518-28-5View Details
Podophyllotoxin 95.00% CAS 518-28-5View Details
518-28-5 -
 Podophyllotoxin 98% CAS 518-28-5View Details
Podophyllotoxin 98% CAS 518-28-5View Details
518-28-5 -
 Podophyllotoxin 90% CAS 518-28-5View Details
Podophyllotoxin 90% CAS 518-28-5View Details
518-28-5 -
 Podophyllotoxin CAS 518-28-5View Details
Podophyllotoxin CAS 518-28-5View Details
518-28-5 -
 Podophyllotoxin >98% (HPLC) CAS 518-28-5View Details
Podophyllotoxin >98% (HPLC) CAS 518-28-5View Details
518-28-5 -
 Podophyllotoxin CAS 518-28-5View Details
Podophyllotoxin CAS 518-28-5View Details
518-28-5
