Felbamate
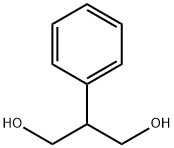
Synonym(s):2-Phenyl-1,3-propanediol dicarbamate
- CAS NO.:25451-15-4
- Empirical Formula: C11H14N2O4
- Molecular Weight: 238.24
- MDL number: MFCD00865296
- EINECS: 247-001-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-21 22:41:43
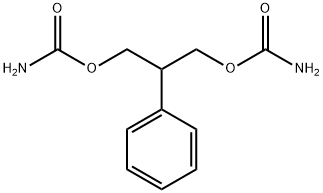
What is Felbamate?
Absorption
>90%
Toxicity
LD50=5000 mg/kg (Orally in rats)
Description
Felbamate, characterized by its low toxicity and wide margin of safety, is efficacious in treating refractory patients with generalized tonic-clonic and complex partial seizures as monotherapy and adjunctive therapy.It has also been demonstrated to have a neuroprotective effect in cerebral ischemia and hypoxia. It has been suggested that the mechanism of its anticonvulsant activity is possibly through an interaction with the strychnineinsensitive receptor site on the NMDA receptor complex.
Chemical properties
White Powder. Solubility in water 0.33 mg/mL, in ethanol 5.0 mg/mL, and in DMF 333.4 mg/mL.
Originator
Carter-Wallace (U.S.A.)
The Uses of Felbamate
Antiepileptic, structurally similar to meprobamate
The Uses of Felbamate
Antiepileptic, structurally similar to meprobamate.
Background
Felbamate is an anticonvulsant drug used in the treatment of epilepsy. In particular, in the adult patient population, it can be employed to treat partial seizures (with and without generalization). Alternatively, it is used to treat partial and generalized seizures associated with Lennox-Gastaut syndrome in children. It has a weak inhibitory effect on GABA receptor binding sites.
Indications
For use only in those patients who respond inadequately to alternative treatments and whose epilepsy is so severe that a substantial risk of aplastic anemia and/or liver failure is deemed acceptable in light of the benefits conferred by its use.
What are the applications of Application
Felbamate is an antagonist at the NMDA-associated glycine binding site
Definition
ChEBI: The bis(carbamate ester) of 2-phenylpropane-1,3-diol. An anticonvulsant, it is used in the treatment of epilepsy.
brand name
Felbatol
Biological Functions
Felbamate (Felbatol) was introduced with the expectation
that it would become a major drug in the treatment
of epilepsy. Felbamate exhibited few manifestations of
serious toxicity in early clinical trials. Soon after its introduction,
however, it became apparent that its use was
associated with a high incidence of aplastic anemia.
Consequently, felbamate is indicated only for patients
whose epilepsy is so severe that the risk of aplastic anemia
is considered acceptable.
While its mechanism of action has not been clearly
established, felbamate shows some activity as an inhibitor
of voltage-dependent sodium channels in a manner
similar to that of phenytoin and carbamazepine.
Felbamate also interacts at the strychnine-insensitive
glycine recognition site on the NMDA receptor–
ionophore complex.Whether this effect is important to
its anticonvulsant activity is not clear.
Hazard
Low toxicity by ingestion. Human systemic effects.
Biological Activity
Anticonvulsant, acting as an antagonist at the NMDA-associated glycine binding site.
Biochem/physiol Actions
Anticonvulsant agent that is an allosteric antagonist at the NR2B subunit of the NMDA glutamate receptor; also has γ-aminobutyric acid (GABAA) receptor agonist properties.
Mechanism of action
Gabapentin is a water-soluble amino acid originally designed to be a GABA-mimetic analogue capable of penetrating the CNS.
Surprisingly, it has no direct GABA-mimetic activity, nor is it active on sodium channels. The mechanism of action remains
unknown, although it has been suggested that gabapentin may alter the metabolism or release of GABA. Gabapentin
raises brain GABA levels in patients with epilepsy. Recent studies have demonstrated gabapentin binding to calcium
channels in a manner that can be allosterically modulated.
Gabapentin is indicated as an adjunct for use against partial seizures with or without secondary generalization, in patients
older than 12 years, and as adjunct for the treatment of partial seizures in children 3 to 12 years of age. It also is approved for
the treatment of postherpetic neuralgia.
Pharmacokinetics
Felbamate is an antiepileptic indicated as monotherapy or as an adjunct to other anticonvulsants for the treatment of partial seizures resulting from epilepsy. Receptor-binding studies in vitro indicate that felbamate has weak inhibitory effects on GABA-receptor binding, benzodiazepine receptor binding, and is devoid of activity at the MK-801 receptor binding site of the NMDA receptor-ionophore complex. However, felbamate does interact as an antagonist at the strychnine-insensitive glycine recognition site of the NMDA receptor-ionophore complex.
Pharmacokinetics
The pharmacokinetic properties for gabapentin generally are favorable, with a bioavailability of 60% when given in low doses and somewhat less when given at higher doses because of saturable intestinal uptake by the L-amino-acid transporter. The L-amino-acid transporter is very susceptible to substrate saturation (low Km value). Its absorption and distribution into the CNS appears to be dependent on this amino acid transporter. Following the administration of an oral dose, gabapentin reaches peak plasma concentration in 2 to 3 hours. Additionally, it exhibits linear pharmacokinetics. Moreover, it is not extensively metabolized, nor is it an inducer of hepatic metabolizing enzymes. The elimination of unmetabolized gabapentin occurs by the renal route. Although its therapeutic range is not well characterized, gabapentin has a broad therapeutic index. This implies that a wide range of doses can be used, based on individual patient needs, without significant limitation because of dose-dependent side effects. Protein binding is negligible. Its elimination half-life of 5 to 7 hours is not affected by the dose or by other drugs, and its short half-life necessitates multiple daily administration.
Clinical Use
Felbamate is a dicarbamate that is structurally similar to the antianxiety drug meprobamate. It was approved by the U.S. FDA for antiseizure use in 1993. Following the occurrence of rare cases of aplastic anemia and of severe hepatotoxicity associated with the use of felbamate during early 1994, however, a black box warning was added to the drug's package insert). Despite this, felbamate continues to be used in many patients, although not as a first-line treatment. These toxicity effects may be attributed to the formation of toxic metabolites. Although felbamate use is now uncommon, it is used for severe refractory seizures, either partial, myoclonic, or atonic, or in Lennox-Gastaut syndrome
Side Effects
Adverse effects of gabapentin are uncommon and not serious. The CNS effects include mild to moderate sedation, fatigue,
ataxia, headache, dizziness, and diplopia. Gabapentin may exacerbate myoclonus, but the effect is mild and does not require
discontinuance of the drug. It has been associated with the development of neuropsychiatric adverse events in
children.
Drug interactions are infrequent with gabapentin. It does not induce hepatic metabolizing enzymes, nor do other AEDs affect its
metabolism and elimination. Antacids may decrease absorption. Gabapentin dosage may need to be decreased in patients with
renal disease or in the elderly.
Synthesis
The synthesis of felbamate was first published in 1959. 2-Phenyl-1,3-propanediole is reacted with ethylcarbamate to yield felbamate.
Veterinary Drugs and Treatments
Felbamate is an anticonvulsant agent that may useful for treating seizure disorders (especially complex partial seizures) in dogs. A potential advantage of felbamate therapy is that when used alone or in combination with phenobarbital and/or bromides, it does not appear to cause additive sedation.
Metabolism
Hepatic
Metabolism
Although the metabolism of felbamate has not been fully characterized, felbamate is esterase hydrolyzed to its monocarbamate metabolite, 2-phenyl-1,3-propanediol monocarbamate, which subsequently is oxidized via aldehyde dehydrogenase to its major human metabolite 3-carbamoyl-2-phenylpropionic acid. Other metabolites include the p-hydroxy and mercapturic acid metabolites of felbamate, which have been identified in human urine. Felbamate is a substrate for CYP2C19, with minor activity for CYP3A4 and CYP2E1. Thompson et al. has provided evidence for the formation of the reactive metabolite, 3-carbamoyl-2-phenylpropionaldehyde (CBMA), from the alcohol oxidation of 2-phenyl-1,3-propanediol monocarbamate. CBMA then undergoes spontaneous elimination to another reactive intermediate, 2-phenylpropenal (more commonly known as atropaldehyde), which is proposed to play a role in the development of toxicity during felbamate therapy. CBMA or a further product has been shown to provoke an immune response in mice. Evidence for in vivo atropaldehyde formation was confirmed with the identification of its mercapturic acid conjugates in human urine after felbamate administration. This is consistent with the hypothesis that atropaldehyde reacts rapidly with thiol nucleophiles, such as glutathione, to form mercapturates. More recently, a fluorine analogue of felbamate was synthesized in which the benzylic C2 hydrogen of the propane chain was replaced with fluorine, preventing the formation of atropaldehyde and confirming that the acidic benzylic hydrogen plays a pivotal role in its formation. This analogue is presently undergoing drug development. Felbamate administration exhibited linear kinetics, with a half-life of 20 to 23 hours in the absence of enzyme-inducing AEDs. Approximately 50% of an oral dose of felbamate is excreted unchanged.
Properties of Felbamate
| Melting point: | 148-1500C |
| Boiling point: | 511.9±50.0 °C(Predicted) |
| Density | 1.275±0.06 g/cm3(Predicted) |
| Flash point: | 9℃ |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | alcohol: soluble |
| form | Solid |
| pka | 12.99±0.50(Predicted) |
| color | White |
| CAS DataBase Reference | 25451-15-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Felbamate(25451-15-4) |
Safety information for Felbamate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H370:Specific target organ toxicity, single exposure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P311:Call a POISON CENTER or doctor/physician. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Felbamate
Felbamate manufacturer
Solara Active Pharma Sciences Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 25451-15-4 Felbamate 99%View Details
25451-15-4 Felbamate 99%View Details
25451-15-4 -
 25451-15-4 96%View Details
25451-15-4 96%View Details
25451-15-4 -
 25451-15-4 98%View Details
25451-15-4 98%View Details
25451-15-4 -
 Felbamate 25451-15-4 98%View Details
Felbamate 25451-15-4 98%View Details
25451-15-4 -
 Felbamate 98%View Details
Felbamate 98%View Details
25451-15-4 -
 25451-15-4 Felbamate 98%View Details
25451-15-4 Felbamate 98%View Details
25451-15-4 -
 Felbamate 95.00% CAS 25451-15-4View Details
Felbamate 95.00% CAS 25451-15-4View Details
25451-15-4 -
 Felbamate CAS 25451-15-4View Details
Felbamate CAS 25451-15-4View Details
25451-15-4