VIDARABINE (200 MG)G-2939UG/MG(AI)
- CAS NO.:24356-66-9
- Empirical Formula: C10H15N5O5
- Molecular Weight: 285.2566
- MDL number: MFCD00150980
- EINECS: 678-339-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-18 11:31:14
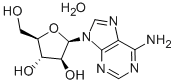
What is VIDARABINE (200 MG)G-2939UG/MG(AI)?
Absorption
Systemetic absorption of vidarabine should not be expected to occur following ocular administration and swallowing lacrimal secretions.
Toxicity
Acute massive overdosage by oral ingestion of the ophthalmic ointment has not occurred. However, the rapid deamination to arabinosylhypoxanthine should preclude any difficulty. The oral LD50 for vidarabine is greater than 5020 mg/kg in mice and rats. No untoward effects should result from ingestion of the entire contents of the tube. Overdosage by ocular instillation is unlikely because any excess should be quickly expelled from the conjunctival sac.
The Uses of VIDARABINE (200 MG)G-2939UG/MG(AI)
Antiviral.
Background
A nucleoside antibiotic isolated from Streptomyces antibioticus. It has some antineoplastic properties and has broad spectrum activity against DNA viruses in cell cultures and significant antiviral activity against infections caused by a variety of viruses such as the herpes viruses, the vaccinia VIRUS and varicella zoster virus.
Indications
For treatment of chickenpox - varicella, herpes zoster and herpes simplex
Definition
A biologically active pharmaceu- tical product having both antitumor and antiviral properties. It was originally prepared (1959) by chemical synthesis at Stanford Research Institute, and later isolated from a fermentation beer of Strep- tomyces antibioticus.
brand name
Vira-A (Parkdale).
Pharmacokinetics
Vidarabine is a synthetic purine nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and varicella-zoster virus (VZV). The inhibitory activity of Vidarabine is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts Vidarabine into Vidarabine monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In vitro, Vidarabine triphosphate stops the DNA replication of herpes virus by being incorporated into the DNA strand and preventing the formation of phosphodiester bridges between bases. This ultimately leads to destabilization of the viral DNA strands.
Metabolism
In laboratory animals, vidarabine is rapidly deaminated in the gastrointestinal tract to Ara-Hx.
Properties of VIDARABINE (200 MG)G-2939UG/MG(AI)
| Melting point: | 253 °C |
| Boiling point: | 427.69°C (rough estimate) |
| Density | 1.3439 (rough estimate) |
| refractive index | -3.0 ° (C=1, DMF) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | soluble in Dimethylformamide |
| form | powder to crystal |
| color | White to Almost white |
| Water Solubility | 513.5mg/L(temperature not stated) |
| Merck | 14,9976 |
Safety information for VIDARABINE (200 MG)G-2939UG/MG(AI)
Computed Descriptors for VIDARABINE (200 MG)G-2939UG/MG(AI)
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


![ADENINE-BETA-D-ARABINOFURANOSIDE 5'-MONOPHOSPHATE, DIAMMONIUM SALT, [2,8-3H]-](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB0265278.gif)


You may like
-
 Vidarabine Monohydrate CAS 24356-66-9View Details
Vidarabine Monohydrate CAS 24356-66-9View Details
24356-66-9 -
 Vidarabine monohydrate 95% CAS 24356-66-9View Details
Vidarabine monohydrate 95% CAS 24356-66-9View Details
24356-66-9 -
 Vidarabine monohydrate CAS 24356-66-9View Details
Vidarabine monohydrate CAS 24356-66-9View Details
24356-66-9 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4