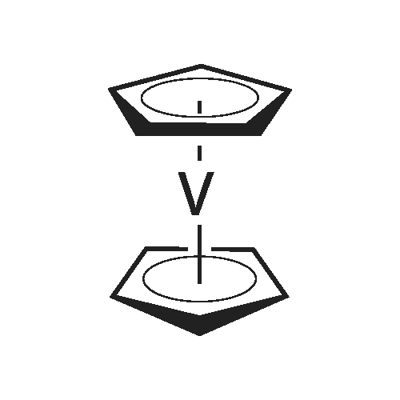
Vanadinocene Dichloride
Synonym(s):Di-π-Cyclopentadienylvanadium dichloride;Dichlorobis(cyclopentadienyl)vanadium;Dichlorovanadocene;Vanadocene dichloride
- CAS NO.:12083-48-6
- Empirical Formula: C10H10Cl2V10*
- Molecular Weight: 252.03
- MDL number: MFCD00014260
- EINECS: 235-150-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-17 17:11:57
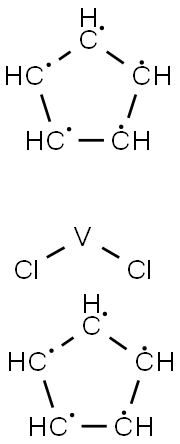
What is Vanadinocene Dichloride?
Chemical properties
dark green crystalline powder
The Uses of Vanadinocene Dichloride
Vanadocene dichloride is used like other metallocenes as a catalyst, ultraviolet absorber, reducing agent, free radical scavenger, antiknock agent in gasoline, and as a developmental anticancer drug.
The Uses of Vanadinocene Dichloride
Bis(cyclopentadienyl)vanadium(IV) dichloride is a catalyst for solvent free, room temperature preparation of vicinal amino alcohols as fungistatic agents, catalyst fot oligomerization reactions via the Aufbau propagation reaction and as a catalyst for aqueous polymerization reactions using oxygen as a co catalyst. It is used as a catalyst for dl-selective pinacol-type coupling reactions, as an affecter of crystal growth of calcium carbonate and and as an inhibitor of human topoisomerase II enzyme with antitumor activity.
What are the applications of Application
Bis(cyclopentadienyl)vanadium(IV) dichloride is a useful catalyst
General Description
Pale green crystals or green powder.
Air & Water Reactions
Extremely sensitive to exposure to air. Also extremely sensitive to moisture; decomposes in water
Reactivity Profile
Organometallics, such as BIS(CYCLOPENTADIENYL)VANADIUM DICHLORIDE, are reactive with many other groups. Incompatible with acids and bases. Organometallics are good reducing agents and therefore incompatible with oxidizing agents. Often reactive with water to generate toxic or flammable gases. Organometallics containing halogens (fluorine, chlorine, bromine, iodine) bonded to the metal typically with generate gaseous hydrohalic acids (HF, HCl, HBr, HI) with water.
Fire Hazard
Flash point data for BIS(CYCLOPENTADIENYL)VANADIUM DICHLORIDE are not available. BIS(CYCLOPENTADIENYL)VANADIUM DICHLORIDE is probably combustible.
Pharmaceutical Applications
Vanadocene dichloride [( η5-C5H5)2VCl2, dichloro bis(η5-cyclopentadienyl)vanadium(IV)] is structurally very similar to Cp2TiCl2. It also consists of a metal centre with an oxidation number of +IV, in this case vanadium, and two Cp and two chloride ligands. Vanadocene dichloride is a 17-electron complex containing an unpaired electron and is therefore paramagnetic .
Vanadocene dichloride has found application as a catalyst for polymerisation reactions, but was also intensively studied as an anticancer agent in parallel to Cp2TiCl2 because of their structural similarities. Vanadocene dichloride has proven to be even more effective than its titanium analogue as an antiproliferative agent against both animal and human cell lines in preclinical testing. The main problems are the difficult characterisation of the active vanadium compounds and their fast hydrolysis.
Pharmaceutical Applications
Vanadocene dichloride [(η5-C5H5)2VCl2, dichloro bis(η5-cyclopentadienyl)vanadium(IV)] is structurally very similar to Cp2TiCl2. It also consists of a metal centre with an oxidation number of +IV, in this case vanadium, and two Cp and two chloride ligands. Vanadocene dichloride is a 17-electron complex containing an unpaired electron and is therefore paramagnetic. Vanadocene dichloride has found application as a catalyst for polymerisation reactions, but was also intensively studied as an anticancer agent in parallel to Cp2TiCl2 because of their structural similarities. Vanadocene dichloride has proven to be even more effective than its titanium analogue as an antiproliferative agent against both animal and human cell lines in preclinical testing.
Properties of Vanadinocene Dichloride
| Melting point: | 182-194 °C (dec.)(lit.) |
| Density | 1,6 g/cm3 |
| storage temp. | 2-8°C |
| solubility | soluble in H2O, chloroform, ethanol |
| form | crystal |
| color | green |
| Specific Gravity | 1.6 |
| Water Solubility | Reacts with water |
| Sensitive | Air & Moisture Sensitive |
| Exposure limits | NIOSH: Ceiling 0.05 mg/m3 |
| CAS DataBase Reference | 12083-48-6(CAS DataBase Reference) |
| EPA Substance Registry System | Vanadocene dichloride (12083-48-6) |
Safety information for Vanadinocene Dichloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Vanadinocene Dichloride
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

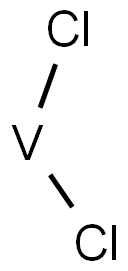

You may like
-
 Vanadinocene Dichloride CAS 12083-48-6View Details
Vanadinocene Dichloride CAS 12083-48-6View Details
12083-48-6 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4