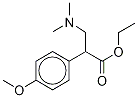
Venlafaxine
- CAS NO.:93413-69-5
- Empirical Formula: C17H27NO2
- Molecular Weight: 277.4
- MDL number: MFCD00864385
- EINECS: 618-944-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
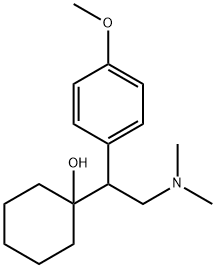
What is Venlafaxine?
Absorption
Venlafaxine is well absorbed after oral administration with an absolute bioavailability of approximately 45%. In mass balance studies, at least 92% of a single oral dose of venlafaxine was absorbed. After twice-daily oral administration of immediate-release formulation of 150 mg venlafaxine, Cmax was 150 ng/mL and Tmax was 5.5 hours. Cmax and Tmax of ODV were 260 ng/mL and nine hours, respectively. The extended-release formulation of venlafaxine has a slower rate of absorption, but the same extent of absorption as the immediate-release formulation. After once-daily administration of extended-release formulation of 75 mg venlafaxine, Cmax was 225 ng/mL and Tmax was two hours. Cmax and Tmax of ODV were 290 ng/mL and three hours, respectively.
Food does not affect the bioavailability of venlafaxine or its active metabolite, O-desmethylvenlafaxine (ODV).
Toxicity
Oral LD50 was 350 mg/kg in female rats and 700 mg/kg in male rats.
There are reports of acute overdosage with venlafaxine either alone or in combination with other drugs including alcohol. Doses up to several-fold higher than the usual therapeutic dose have been ingested in these cases of acute overdosage. Somnolence is the most commonly reported symptom, along with other symptoms such as paresthesia of the extremities, moderate dizziness, altered consciousness, nausea, vomiting, numb hands and feet, hot-cold spells (which occur a few days after the overdose event), hypotension, convulsions, sinus and ventricular tachycardia, rhabdomyolysis, vertigo, liver necrosis, electrocardiogram changes (e.g., prolongation of QT interval, bundle branch block, QRS prolongation), serotonin syndrome, and death.
There is no known antidote for venlafaxine overdose. Cases of overdose have been managed with or without symptomatic treatment, hospitalization, and activated charcoal.
Retrospective studies suggest that the risk of fatal outcomes from venlafaxine overdosage is higher than that of SSRI antidepressants, but lower than that of tricyclic antidepressants.
Chemical properties
White Solid
The Uses of Venlafaxine
A optically active version of Venlafaxine, a selective serotonin noradrenaline reuptake inhibitor. Used as an antidepressant
The Uses of Venlafaxine
antidepressant;serotonin-norepinephrine reuptake inhibitor
The Uses of Venlafaxine
A selective serotonin noradrenaline reuptake inhibitor. Used as an antidepressant.
What are the applications of Application
D,L-Venlafaxine is an isomer of Venlafaxine
Background
Venlafaxine is an antidepressant and a serotonin and norepinephrine reuptake inhibitor (SNRI). Its active metabolite, desvenlafaxine, works by blocking the reuptake of serotonin and norepinephrine, which are key neurotransmitters in mood regulation. Venlafaxine is officially approved to treat major depressive disorder (MDD), generalized anxiety disorder (GAD), social anxiety disorder, and panic disorder in adults. The immediate formulation of the drug, marketed as Effexor, was first approved by the FDA in 1993 and the extended-release formulation, Effexor XR, was later introduced in 1997.
Venlafaxine has been used as a first-line treatment for MDD, GAD, social anxiety disorder, and panic disorder in Canada for many years. It was also considered a second-line treatment for obsessive-compulsive disorder (OCD). Venlafaxine was also investigated in off-label uses for the prophylaxis of migraine headaches, for reduction of vasomotor symptoms associated with menopause, and for the management of neuropathic pain (although there is only minimal evidence of efficacy for this condition).
Indications
Venlafaxine is indicated for the management of major depressive disorder (MDD), generalized anxiety disorder (GAD), social anxiety disorder (SAD), and panic disorder.
Definition
ChEBI: A tertiary amino compound that is N,N-dimethylethanamine substituted at position 1 by a 1-hydroxycyclohexyl and 4-methoxyphenyl group.
brand name
Effexor (Wyeth).
Biological Functions
Venlafaxine (Effexor) inhibits the reuptake of both serotonin and norepinephrine at their respective presynaptic sites.This drug does not have significant effects at muscarinic, histamine, or α-adrenergic receptors and therefore is devoid of many of the side effects associated with the TCAs.Venlafaxine and its active metabolite O-desmethyl-venlafaxine, have half lives of 5 and 11 hours respectively, so dosing twice a day is necessary. However, an extended release preparation (Effexor XR) now allows for once-daily dosing and better tolerance. Venlafaxine has a side effect profile similar to that of the SSRIs. Higher doses of venlafaxine result in modest increases in blood pressure in approximately 5% of patients.Venlafaxine has minimal effects on the cytochrome P450 enzyme system.
General Description
The structure and activity of venlafaxine (Effexor) are in accordwith the general SARs for the group. As expected, it isan effective antidepressant. Venlafaxine is a serotonin–norepinephrinereuptake inhibitor (SNRI).
Pharmaceutical Applications
Venlafaxine is a serotonin and noradrenalin reuptake inhibitor (SNRI) and is used as an antidepressant. Compared to tricyclic antidepressants, it lacks the antimuscarinic and sedative side effects. Nevertheless, treatment with venlafaxine can lead to a higher risk of withdrawal symptoms.
Mechanism of action
Venlafaxine and its active metabolite, O-desmethylvenlafaxine (ODV), have dual mechanisms of action, with
preferential affinity for 5-HT reuptake and weak inhibition of NE and dopamine reuptake. Venlafaxine is
approximately 30 times more potent as an inhibitor of SERT than of NET. Because of the 30 times
difference in transporter affinities, increasing the dose of venlafaxine from 75 to 375 mg/day can sequentially
inhibit SERT and NERT . Thus, venlafaxine displays an ascending dose-dependent antidepressant response
in contrast to the flat dose–antidepressant response curve observed with the SSRIs. This sequential action
for venlafaxine also is consistent with its dose-dependent adverse-effect profile. Its mechanism of action is
similar to imipramine.
Venlafaxine is rapidly and well absorbed, but with a bioavailability of 45%, which has been attributed to
first-pass metabolism. Food delays its absorption but does not impair the extent of absorption.
Venlafaxine is distributed into breast milk. Venlafaxine is primarily metabolized in the liver by CYP2D6 to its
primary metabolite, ODV, which is approximately equivalent in pharmacological activity and potency to venlafaxine. In vitro studies indicate that CYP3A4 also is involved in the metabolism of venlafaxine to its
minor and less active metabolite, N-desmethylvenlafaxine. Protein binding for venlafaxine and
ODV is low and is not a problem for drug interactions. In patients with hepatic impairment, elimination
half-lives were increased by approximately 30% for venlafaxine and approximately 60% for ODV. In patients with renal function impairment, elimination half-lives were increased by approximately 40
to 50% for venlafaxine and for ODV. At steady-state doses, venlafaxine and ODV exhibit dose-proportional
linear pharmacokinetics over the dose range of 75 to 450 mg/day. Steady-state concentrations of venlafaxine
and ODV are attained within 3 days with regular oral dosing. Venlafaxine and its metabolites are excreted
primarily in the urine (87%).
Pharmacokinetics
Venlafaxine is an antidepressant agent that works to ameliorate the symptoms of various psychiatric disorders by increasing the level of neurotransmitters in the synapse. Venlafaxine does not mediate muscarinic, histaminergic, or adrenergic effects.
Clinical Use
Venlafaxine is a methoxyphenylethylamine antidepressant that resembles an open TCA with one of the aromatic rings replaced by a cyclohexanol ring and a dimethylaminomethyl group rather than a dimethylaminopropyl chain.
Side Effects
The potential for cardiotoxicity with venlafaxine during normal use and for various toxicities in overdose
situations are key concerns. Venlafaxine displays minimal in vitro affinity for the other neural neurotransmitter
receptors and, thus, a low probability for adverse effects. To minimize GI upset (e.g., nausea), venlafaxine
can be taken with food without affecting its GI absorption. Venlafaxine should be administered as a single
daily dose with food at approximately the same time each day. The extended-release capsules should be
swallowed whole with fluid and should not be divided, crushed, chewed, or placed in water.
Whenever venlafaxine is being discontinued after more than 1 week of therapy, it generally is recommended
that the patient be closely monitored and the dosage of the drug be tapered gradually to reduce the risk of
withdrawal symptoms.
Although venlafaxine is a weak inhibitor of CYP2D6, variability has been observed in the pharmacokinetic
parameters of venlafaxine in patients with hepatic or renal function impairment. As a precaution, elderly
patients taking venlafaxine concurrently with a drug that has a narrow therapeutic index and also is
metabolized by CYP2D6 should be carefully monitored. Concurrent use of CYP3A4 inhibitors with venlafaxine
has been shown to interfere with its metabolism and clearance. Similar to the other antidepressants that block
5-HT reuptake, venlafaxine may interact pharmacodynamically to cause toxic levels of 5-HT to accumulate,
leading to the 5-HT syndrome.
Drug interactions
Potentially hazardous interactions with other drugs
Analgesics: increased risk of bleeding with aspirin
and NSAIDs; possibly increased serotonergic effects
with tramadol.
Anti-arrhythmics: risk of ventricular arrhythmias
with amiodarone - avoid.
Antibacterials: risk of ventricular arrhythmias with
erythromycin, moxifloxacin - avoid.
Anticoagulants: effects of warfarin possibly
enhanced; possibly increased risk of bleeding with
dabigatran.
Antidepressants: avoid with MAOIs and
moclobemide (increased risk of toxicity); possibly
enhanced serotonergic effects with duloxetine,
mirtazapine and St John’s wort; possible increased
risk of convulsions with vortioxetine - avoid.
Antimalarials: avoid concomitant use with
artemether/lumefantrine and piperaquine with
artenimol.
Antipsychotics: increases concentration of clozapine
and haloperidol.
Beta-blockers: risk of ventricular arrhythmias with
sotalol - avoid.
Dapoxetine: possible increased risk of serotonergic
effects - avoid.
Dopaminergics: use entacapone with caution;
increased risk of hypertension and CNS excitation
with selegiline - avoid concomitant use.
Methylthioninium: risk of CNS toxicity - avoid if
possible.
Metabolism
Following absorption, venlafaxine undergoes extensive presystemic metabolism in the liver. It primarily undergoes CYP2D6-mediated demethylation to form its active metabolite O-desmethylvenlafaxine (ODV). Venlafaxine can also undergo N-demethylation mediated by CYP2C9, and CYP2C19, and CYP3A4 to form N-desmethylvenlafaxine (NDV) but this is a minor metabolic pathway. ODV and NDV further metabolized by CYP2C19, CYP2D6 and/or CYP3A4 to form N,O-didesmethylvenlafaxine (NODV) and NODV can be further metabolized to form N, N, O-tridesmethylvenlafaxine, followed by a possible glucuronidation.
Metabolism
Venlafaxine undergoes extensive first-pass
metabolism in the liver mainly to the active
metabolite O-desmethylvenlafaxine; this is mediated
by the cytochrome P450 isoenzyme CYP2D6.
The isoenzyme CYP3A4 is also involved in the
metabolism of venlafaxine. Other metabolites
include N-desmethylvenlafaxine and N,Odidesmethylvenlafaxine. Peak plasma concentrations of
venlafaxine and O-desmethylvenlafaxine occur about 2
and 4 hours after a dose, respectively.
The majority of venlafaxine is excreted in the urine,
mainly in the form of its metabolites, either free or in
conjugated form.
Properties of Venlafaxine
| Melting point: | 72-74°C |
| Boiling point: | 397.6±27.0 °C(Predicted) |
| Density | 1.060±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | soluble in Dichloromethane, Ethyl Acetate |
| form | Crystalline |
| pka | pKa 9.5 (Uncertain) |
| color | White to off-white |
| Water Solubility | <0.1g/L(room temperature) |
| CAS DataBase Reference | 93413-69-5(CAS DataBase Reference) |
Safety information for Venlafaxine
Computed Descriptors for Venlafaxine
Venlafaxine manufacturer
Gangwal Healthcare Pvt Ltd
Innovassynth Technologies (I) Ltd.
SLS Pharmaceuticals Private Limited
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 90300-78-4 Venlafaxin HCl 98%View Details
90300-78-4 Venlafaxin HCl 98%View Details
90300-78-4 -
 93413-69-5 98%View Details
93413-69-5 98%View Details
93413-69-5 -
 Venlafaxine 98%View Details
Venlafaxine 98%View Details
93413-69-5 -
 Venlafaxine 99%View Details
Venlafaxine 99%View Details -
 Venlafaxine 93413-69-5 98%View Details
Venlafaxine 93413-69-5 98%View Details
93413-69-5 -
 Venlafaxine 93413-69-5 98%View Details
Venlafaxine 93413-69-5 98%View Details
93413-69-5 -
 93413-69-5 Venlafaxine HCL SR Pellets 98%View Details
93413-69-5 Venlafaxine HCL SR Pellets 98%View Details
93413-69-5 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1