VANADIUM(V) TRICHLORIDE OXIDE
Synonym(s):Trichlorooxovanadium oxide;Vanadium(V) oxytrichloride;Vanadyl trichloride
- CAS NO.:7727-18-6
- Empirical Formula: Cl3OV
- Molecular Weight: 173.3
- MDL number: MFCD00011459
- EINECS: 231-780-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32

What is VANADIUM(V) TRICHLORIDE OXIDE?
Chemical properties
Lemon-yellow liquid. Nonionizing solvent. Dissolves most nonmetals; dissolves and/or reacts with many organic compounds; hydrolyzes in moisture.
Chemical properties
Vanadium(V) oxytrichloride (VOCl3) is readily hydrolyzed and forms coordination compounds with simple donor molecules, eg, ethers, but is reduced by reaction with sulfur-containing ligands and molecules. It is completely miscible with many hydrocarbons and nonpolar metal halides, eg, TiCl4, and it dissolves sulfur.
The Uses of VANADIUM(V) TRICHLORIDE OXIDE
Vanadium(V) trichloride oxide is used as a catalyst in olefin polymerization reaction, especially for the production of ethylene-propylene rubber. It is also used to synthesis organovanadium compounds and as a mordant in dyeing. It acts as a precursor to prepare vanadium pentoxide, vanadyl dichloride and dioxovanadium monochloride. Further, it serves as a reagent and a strong oxidizing agent in organic synthesis.
The Uses of VANADIUM(V) TRICHLORIDE OXIDE
Catalyst in olefin polymerization (ethylenepropylene rubber) organovanadium synthesis.
What are the applications of Application
Vanadium(V) oxychloride is an inorganic compound used for chemical synthesis
Air & Water Reactions
Fumes in air. Reacts with moist air to form vanadic acid and hydrochloric acid fumes [Von Schwartz 1918. p.321]. Reacts exothermically with water to generate acidic fumes and acidic solutions. Violently hygroscopic.
Reactivity Profile
VANADIUM(V) TRICHLORIDE OXIDE is incompatible with bases, including amines, with strong oxidizing agents, and with alcohols. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291]. Combination with sodium metal led to a violent explosion [Bretherick 5th ed., 1995].
Health Hazard
Inhalation of vapor causes irritation of nose and throat. Ingestion causes irritation of mouth and stomach. Contact with eyes or skin causes severe irritation.
Fire Hazard
Special Hazards of Combustion Products: Irritating fumes of hydrogen chloride may form in fires.
Flammability and Explosibility
Non flammable
Safety Profile
Poison by ingestion. A corrosive irritant to skin, eyes, and mucous membranes. Explosive reaction with sodmm. Violently hygroscopic. Violent reaction with rubidium (at 6OoC), potassium. When heated to decomposition it emits toxic fumes of VOx and Cl-. See also VANADIUM COMPOUNDS and HYDROCHLORIC ACID.
Purification Methods
VOCl3 should be lemon yellow in colour. If it is red, it may contain VCl4 and Cl2. Fractionally distil it, and then redistil it over metallic Na, but be careful to leave some residue because the residue can become EXPLOSIVE in the presence of the metal. USE A SAFETY SHIELD and avoid contact with moisture. It readily hydrolyses to vanadic acid and HCl. Store it in a tightly closed container or in sealed ampoules under N2. [Brown & Griffitts Inorg Synth I 106 1939, Brown & Griffitts Inorg Synth IV 80 1953.]
Properties of VANADIUM(V) TRICHLORIDE OXIDE
| Melting point: | −77 °C(lit.) |
| Boiling point: | 126-127 °C(lit.) |
| Density | 1.84 g/mL at 25 °C(lit.) |
| vapor pressure | 13.8 mm Hg ( 20 °C) |
| refractive index | 1.6300 |
| Flash point: | 126-127°C |
| solubility | Miscible with benzene, dichloromethane and hexane. |
| form | Liquid |
| color | Yellow |
| Specific Gravity | 1.84 |
| Water Solubility | decomposes in H2O to vanadic acid and HCl; soluble methanol, ether, acetone, acids [KIR83] [MER06] |
| Sensitive | Moisture Sensitive |
| Merck | 14,9927 |
| Exposure limits | NIOSH: Ceiling 0.05 mg/m3 |
| Dielectric constant | 3.6(26℃) |
| CAS DataBase Reference | 7727-18-6(CAS DataBase Reference) |
| EPA Substance Registry System | Vanadium, trichlorooxo-, (T-4)- (7727-18-6) |
Safety information for VANADIUM(V) TRICHLORIDE OXIDE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for VANADIUM(V) TRICHLORIDE OXIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
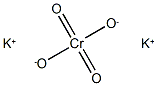
 Vanadium (V) trichloride oxide CAS 7727-18-6View Details
Vanadium (V) trichloride oxide CAS 7727-18-6View Details
7727-18-6 -
 VANADIUM(V)OXYTRICHLORIDE CAS 7727-18-6View Details
VANADIUM(V)OXYTRICHLORIDE CAS 7727-18-6View Details
7727-18-6 -
 Vanadium(V) oxychloride CAS 7727-18-6View Details
Vanadium(V) oxychloride CAS 7727-18-6View Details
7727-18-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1