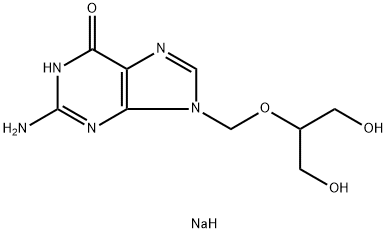
Valganciclovir hydrochloride
Synonym(s):L -Valine 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-3-hydroxypropyl ester monohydrochloride;2-[(2-amino-6-oxo-6,9-dihydro-3H-purin-9-yl)methoxy]-3-hydroxypropyl (2S)-2-amino-3-methylbutanoate hydrochloride hydrate;L-Valine, 2-[(2-amino-1,6-dihydro-6-oxo-9H-purin-9-yl)methoxy]-3-hydroxypropyl ester, monohydrochloride hydrate;Valganciclovir hydrochloride hydrate
- CAS NO.:175865-59-5
- Empirical Formula: C14H23ClN6O5
- Molecular Weight: 390.82
- MDL number: MFCD08460118
- EINECS: 641-360-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
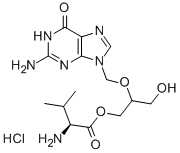
What is Valganciclovir hydrochloride?
Description
Valganciclovir hydrochloride, a prodrug of the antiviral ganciclovir, was launched in the US for the oral treatment of cytemegalovirus (CMV) retinitis, a sight-threatening complication in patients with AIDS. This L-Valyl ester prodrug can be prepared in three steps from the nucleoside analog ganciclovir by trimethylsilyl-protection of the amino group, coupling with N-benzyloxycarbonyl-L-valine-N-carboxyanhydride, hydrolysis with hydrochloric acid and hydrogenolysis of the Cbz-protecting group. Valganciclovir is well absorbed and rapidly hydrolyzed to ganciclovir by intracellular esterases in the intestinal mucosal cells and by hepatic esterases. Unlike ganciclovir, valganciclovir was demonstrated to be actively transported by the intestinal peptide transporter PEPT1 in Caco-2 cells. As a consequence, its absolute bioavailability in human was 10-fold higher compared to ganciclovir (6%). In clinical trials, it was shown that a twice-daily 900 mg dose of valganciclovir resulted in similar systemic ganciclovir exposure to 5 mg/kg twice-daily intravenous injection of ganciclovir. Valganciclovir concentrations could not be quantified in most patients within three to four hours. In a randomized non-blind phase III clinical trial, oral valganciclovir (900 mg twice daily for three weeks then 900 mg once daily for one week) was as effective as intravenous ganciclovir (5 mg/kg twice daily for three weeks then 5 mg/kg once daily). Oral treatment with valganciclovir avoided catheter-related infection that sometimes occurred with intravenous ganciclovir.
Chemical properties
White Crystalline Solid
Originator
Roche (Switzerland)
The Uses of Valganciclovir hydrochloride
Valganciclovir hydrochloride hydrate may be used in HIV-related cell signaling studies.
The Uses of Valganciclovir hydrochloride
A pro-drug of ganciclovir. Used in treatment of retro-virus
The Uses of Valganciclovir hydrochloride
antibacterial, inhibits protein synthesis
The Uses of Valganciclovir hydrochloride
Valganciclovir is the valyl ester prodrug of ganciclovir , an antiviral agent used for the treatment of HIV associated retinitis and for the prevention of post transplant cytomegalovirus (CMV) infections. Upon oral administration, intestinal and hepatic esterases rapidly convert valganciclovir to ganciclovir, which can inhibit viral DNA synthesis (IC50 = 0.95 μM) by targeting the CMV polymerase.
What are the applications of Application
Valganciclovir Hydrochloride is a pro-drug of ganciclovir
brand name
Valcyte (Roche).
General Description
Valganciclovir Hydrochloride is the L-valyl ester of ganciclovir and an antiviral drug, widely used to treat cytomegalovirus infections.
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to pharmacopeia primary standards.
Biochem/physiol Actions
Valganciclovir hydrochloride hydrate is an antiviral used to treat cytomegalovirus infection. It is the prodrug of ganciclovir, a synthetic analog of 2′-deoxy-guanosine which is phosphorylated to a dGTP analog that competitively inhibits the incorporation of dGTP by viral DNA polymerase.
Properties of Valganciclovir hydrochloride
| Melting point: | 162-164°C |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | H2O: ≥8mg/mL |
| form | powder |
| color | white to tan |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 175865-59-5 |
Safety information for Valganciclovir hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H312:Acute toxicity,dermal H332:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P330:Rinse mouth. P363:Wash contaminated clothing before reuse. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P501:Dispose of contents/container to..… |
Computed Descriptors for Valganciclovir hydrochloride
| InChIKey | ZORWARFPXPVJLW-MTFPJWTKSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 175865-59-5 99%View Details
175865-59-5 99%View Details
175865-59-5 -
 175865-59-5 99%View Details
175865-59-5 99%View Details
175865-59-5 -
 175865-59-5 Valganciclovir hydrochloride 98%View Details
175865-59-5 Valganciclovir hydrochloride 98%View Details
175865-59-5 -
 Valganciclovir HCl 98% (HPLC) CAS 175865-59-5View Details
Valganciclovir HCl 98% (HPLC) CAS 175865-59-5View Details
175865-59-5 -
 Valganciclovir Hydrochloride CAS 175865-59-5View Details
Valganciclovir Hydrochloride CAS 175865-59-5View Details
175865-59-5 -
 Valganciclovir Hydrochloride CAS 175865-59-5View Details
Valganciclovir Hydrochloride CAS 175865-59-5View Details
175865-59-5 -
 Valganciclovir hydrochloride CAS 175865-59-5View Details
Valganciclovir hydrochloride CAS 175865-59-5View Details
175865-59-5 -
 Valganciclovir Hydrochloride APIView Details
Valganciclovir Hydrochloride APIView Details
175865-59-5
