Valganciclovir
- CAS NO.:175865-60-8
- Empirical Formula: C14H22N6O5
- Molecular Weight: 354.36
- MDL number: MFCD05662329
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-26 17:04:21
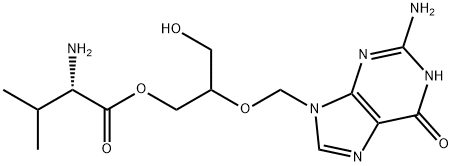
What is Valganciclovir?
Absorption
Valganciclovir is well absorbed from the gastrointestinal tract and the absolute bioavailability from valganciclovir tablets (following administration with food) is approximately 60%.
Toxicity
It is expected that an overdose of valganciclovir could also possibly result in increased renal toxicity.
The Uses of Valganciclovir
Valganciclovir-d8, is the labeled analogue of Valganciclovir (V092000), a pro-drug of Ganciclovir, used in treatment of retro-virus.
The Uses of Valganciclovir
Antiviral.
Indications
Valganciclovir is an antiviral medication used for the treatment of cytomegalovirus infections.
Background
Valganciclovir hydrochloride (Valcyte, manufactured by Roche) is an antiviral medication used to treat cytomegalovirus infections. As the L-valyl ester of ganciclovir, it is actually a prodrug for ganciclovir. After oral administration, it is rapidly converted to ganciclovir by intestinal and hepatic esterases.
Definition
ChEBI: The L-valinyl ester of ganciclovir, into which it is rapidly converted by intestinal and hepatic esterases. It is a synthetic analogue of 2'-deoxyguanosine.
Indications
Valganciclovir (Valcyte) is the L-valyl ester prodrug of ganciclovir.
brand name
Valcyte (Roche).
Pharmacokinetics
Valganciclovir is an antiviral medication used to treat cytomegalovirus infections. As the L-valyl ester of ganciclovir, it is actually a prodrug for ganciclovir. After oral administration, it is rapidly converted to ganciclovir by intestinal and hepatic esterases. After this, it (being an analogue of guanosine) gets incorporated into DNA and thus cannot be properly read by DNA polymerase. This results in the termination of the elongation of viral DNA.
Clinical Use
Oral valganciclovir is comparable to intravenous ganciclovir for the treatment and suppression of CMV retinitis in AIDS patients.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: increased risk of convulsions with
imipenem-cilastatin.
Antivirals: possibly increased didanosine
concentration; profound myelosuppression with
zidovudine - avoid if possible.
Mycophenolate: possibly increased concentrations of
both mycophenolic acid and ganciclovir.
Increased risk of myelosuppression with other
myelosuppressive drugs.
Metabolism
Rapidly hydrolyzed in the intestinal wall and liver to ganciclovir. No other metabolites have been detected.
Metabolism
Valganciclovir is well absorbed from the gastrointestinal tract and rapidly and extensively metabolised in the intestinal wall and liver to ganciclovir. Valganciclovir is eliminated in the urine as unchanged ganciclovir, mainly by glomerular filtration and also active tubular secretion.
Properties of Valganciclovir
| Density | 1.59±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | 9.32±0.20(Predicted) |
| CAS DataBase Reference | 175865-60-8(CAS DataBase Reference) |
Safety information for Valganciclovir
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Valganciclovir
Valganciclovir manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
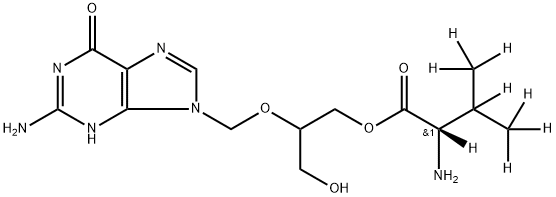
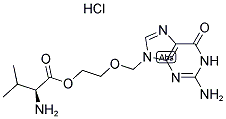



You may like
-
 175865-60-8 Valganciclovir 98%View Details
175865-60-8 Valganciclovir 98%View Details
175865-60-8 -
 175865-60-8 98%View Details
175865-60-8 98%View Details
175865-60-8 -
 Valganciclovir 98%View Details
Valganciclovir 98%View Details
175865-60-8 -
 175865-60-8 Valganciclovir 99%View Details
175865-60-8 Valganciclovir 99%View Details
175865-60-8 -
 175865-60-8 98%View Details
175865-60-8 98%View Details
175865-60-8 -
 Valganciclovir 98%View Details
Valganciclovir 98%View Details
175865-60-8 -
 Valganciclovir HCl 95% CAS 175865-60-8View Details
Valganciclovir HCl 95% CAS 175865-60-8View Details
175865-60-8 -
 Valganciclovir USP powderView Details
Valganciclovir USP powderView Details
175865-59-5
