Ulipristal
- CAS NO.:159811-51-5
- Empirical Formula: C28H35NO3
- Molecular Weight: 433.58
- MDL number: MFCD19443756
- EINECS: 815-032-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-09-21 18:22:39
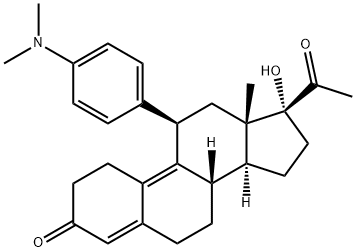
What is Ulipristal?
Absorption
Tmax, healthy subjects, single oral dose = 60-90 minutes; Cmax, healthy subjects, single oral dose = 176 ± 89 ng/mL; AUC(0-∞), healthy subjects, single oral dose = 556 ± 260 ng·h/mL;
Chemical properties
Pale Yellow Solid
The Uses of Ulipristal
Labelled Ulipristal. Ulipristal is a selective progesterone receptor modulator. Ulipristal is used during pregnancy.
The Uses of Ulipristal
Α selective progesterone receptor modulator
The Uses of Ulipristal
Labelled Ulipristal (U700800). Ulipristal is a selective progesterone receptor modulator. Ulipristal is used during pregnancy.
Background
Ulipristal is a selective progesterone receptor modulator used for the purposes of emergency contraception (Ella) and for the treatment of uterine fibroids (Fibristal). It is a derivative of 19-norprogesterone and has both antagonistic and partial agonist activity at the progesterone receptor. It also binds to glucocorticoid receptor, however compared to mifepristone (a progesterone receptor antagonist), ulipristal is more tolerable and has lower glucocorticoid activity and better binding affinity.
Ulipristal is currently recommended as first line therapy for emergency contraception, due to improved efficacy and similar side effect profile as compared to the traditional use of levonorgestrel or the Yuzpe regimen. The exact mechanism of action for ulipristal is still currently debated, though there is evidence that it functions by inhibiting ovulation. A recent systematic review proclaimed that the majority of available evidence demonstrates an inhibitory effect on ovulation rather than a post-fertilization effect on the endometrium, which has been heavily debated due to ethical concerns related to abortion (Rosato et al, 2016). Nevertheless, current and ongoing research into the agent's mechanism of action as an emergency contraceptive continue to provide potentially plausible evidence that ulipristal may, in fact, elicit activity on the endometrium that prevents embryo implantation .
What are the applications of Application
Ulipristal is a selective progesterone receptor modulator used during pregnancy
Indications
As the product Ella (available in Canada and the US), ulipristal is indicated for use as emergency contraception after unprotected intercourse or possible contraceptive failure when administered within 120 hours (5 days) after unprotected intercourse or a known or suspected contraceptive failure. As the product Fibristal (available in Canada), ulipristal is indicated for treatment of the signs and symptoms of uterine fibroids in adult women.
Definition
ChEBI: Ulipristal is a 20-oxo steroid.
Pharmacokinetics
Ulipristal is a selective, reversible progestin receptor modulator and its tissue targets include the uterus, cervix, ovaries, and hypothalamus. Ulipristal may act as an agonist or antagonist in the presence or absence of progesterone based on the tissue target. If given mid-follicular phase, development of the follicle growth is delayed and estradiol concentrations decrease. If given at the time when luteinizing hormone peaks, follicular rapture is delayed by several days. If given early-luteal phase, a decrease in endometrial thickness can be observed.
Metabolism
Ulipristal is metabolized by CYP3A4 and to a lesser extent by CYP1A2 into mono-demethylated (active) and di-methylated (inactive) metabolites.
Properties of Ulipristal
| Boiling point: | 638.5±55.0 °C(Predicted) |
| Density | 1.20 |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 12.94±0.60(Predicted) |
| form | Solid |
| color | Pale Yellow |
Safety information for Ulipristal
Computed Descriptors for Ulipristal
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
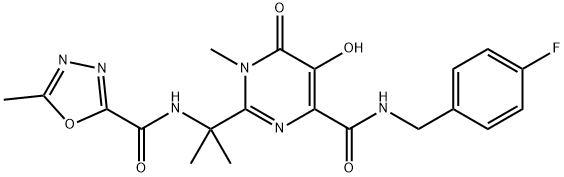
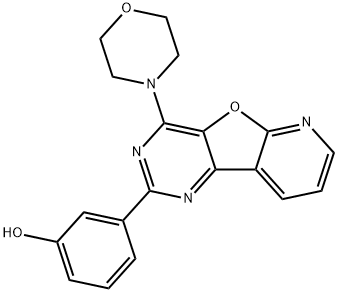
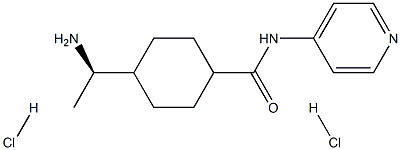
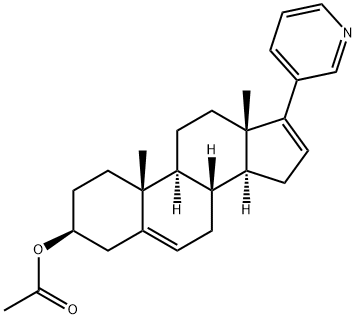
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8