Triisopropylsilyl Trifluoromethanesulfonate
Synonym(s):TIPS triflate;Trifluoromethanesulfonic acid triisopropylsilyl ester;Triisopropylsilyl triflate
- CAS NO.:80522-42-5
- Empirical Formula: C10H21F3O3SSi
- Molecular Weight: 306.42
- MDL number: MFCD00009913
- EINECS: 638-988-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
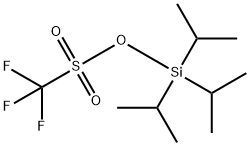
What is Triisopropylsilyl Trifluoromethanesulfonate?
Chemical properties
clear light brown to orange-brown liquid
Physical properties
Colorless oil; bp 83–87 °C/1.7 mmHg; d 1.173 g cm?3.
The Uses of Triisopropylsilyl Trifluoromethanesulfonate
Triisopropylsilyl Trifluoromethanesulfonate is a highly reactive silylating agent and Lewis acid capable of converting primary and secondary alcohols to the corresponding triisopropylsilyl ethers and converting ketones and lactones into their enol silyl ethers; protection of terminal alkynes; promoting conjugate addition of alkynylzinc compounds to α,β-enones; preparation of (triisopropylsilyl)diazomethane, participating in the following (but not limited to) reactions: Silylation of Alcohols, Formations of Enol Silyl Ethers, Alkynyltriisopropylsilanes, Conjugate Addition of Alkynylzinc Bromides, (Triisopropylsilyl)diazomethane, Triisopropylsiloxycarbonyl (Tsoc) and BIPSOP Protecting Groups for Amines, TIPS Protection for Oxazoles/Regioselective Trapping of C-2 Oxazole Anions, Benzannulation Using Triisopropylsilyl Vinyl Ketenes, Cyclization of 1-Silyloxy-1,5-diynes, Desymmetrization of Tartaric Acid Esters etc.
The Uses of Triisopropylsilyl Trifluoromethanesulfonate
Triisopropylsilyl trifluoromethanesulfonate is used as a reagent for the introduction of triisopropylsilyl(TIPS) group in organic synthesis. It is involved in the synthesis of 2-substituted benzothiopyran-4-ones and silyloxy acetylenes. As a protecting group in organic synthesis, it is utilized especially for the protection of primary amines. Furthermore, it plays an important role in Takahashi Taxol total synthesis or for chemical glycosylation reactions.
Preparation
To 38.2 g (0.242 mol) of triisopropylsilane at 0°C under argon is added 23.8 mL (0.266 mol) of trifluoromethanesulfonic acid dropwise. The solution is stirred at 22°C for 16 h, at which time no further hydrogen gas evolves (removed through a bubbler). The resulting product is distilled through a 30-cm vacuum jacketed Vigreux column under reduced pressure: 71.7 g (97% yield) of TIPS triflate; bp 83–87°C/1.7 mmHg.
Properties of Triisopropylsilyl Trifluoromethanesulfonate
| Boiling point: | 45-46 °C/0.03 mmHg |
| Density | 1.14 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 213 °F |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | Miscible with chloroform and ethyl acetate. |
| form | Liquid |
| color | Clear light brown to orange-brown |
| Specific Gravity | 1.14 |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Sensitive | Moisture Sensitive |
| BRN | 3591541 |
| InChI | InChI=1S/C10H21F3O3SSi/c1-7(2)18(8(3)4,9(5)6)16-17(14,15)10(11,12)13/h7-9H,1-6H3 |
| CAS DataBase Reference | 80522-42-5(CAS DataBase Reference) |
Safety information for Triisopropylsilyl Trifluoromethanesulfonate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Triisopropylsilyl Trifluoromethanesulfonate
| InChIKey | LHJCZOXMCGQVDQ-UHFFFAOYSA-N |
| SMILES | C(F)(F)(F)S(O[Si](C(C)C)(C(C)C)C(C)C)(=O)=O |
Triisopropylsilyl Trifluoromethanesulfonate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 80522-42-5 TRIISOPROPYLSILYL TRIFLUOROMETHANESULFONATE TIPS triflate, Triisopropylsilyl triflate 98%View Details
80522-42-5 TRIISOPROPYLSILYL TRIFLUOROMETHANESULFONATE TIPS triflate, Triisopropylsilyl triflate 98%View Details
80522-42-5 -
 80522-42-5 98%View Details
80522-42-5 98%View Details
80522-42-5 -
 Triisopropylsilyl trifluoromethanesulfonate CAS 80522-42-5View Details
Triisopropylsilyl trifluoromethanesulfonate CAS 80522-42-5View Details
80522-42-5 -
 Triisopropylsilyl Trifluoromethanesulfonate CAS 80522-42-5View Details
Triisopropylsilyl Trifluoromethanesulfonate CAS 80522-42-5View Details
80522-42-5 -
 Triisopropylsilyl trifluoromethanesulfonate, 97% CAS 80522-42-5View Details
Triisopropylsilyl trifluoromethanesulfonate, 97% CAS 80522-42-5View Details
80522-42-5 -
 Triisopropylsilyl trifluoromethanesulfonate 96% CAS 80522-42-5View Details
Triisopropylsilyl trifluoromethanesulfonate 96% CAS 80522-42-5View Details
80522-42-5 -
 Triisopropylsilyl trifluoromethanesulfonate CAS 80522-42-5View Details
Triisopropylsilyl trifluoromethanesulfonate CAS 80522-42-5View Details
80522-42-5 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0
