Trimethoxymethane
Synonym(s):Methyl orthoformate, Orthoformic acid trimethyl ester;TMOF;Trimethoxymethane;Trimethyl orthoformate
- CAS NO.:149-73-5
- Empirical Formula: C4H10O3
- Molecular Weight: 106.12
- MDL number: MFCD00008483
- EINECS: 205-745-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
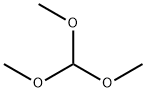
What is Trimethoxymethane?
Description
Trimethyl orthoformate is an effective solvent for thallium(III) nitrate-mediated oxidations. It undergoes acid catalyzed reaction with 6-(N-D-ribitylanilino) uracils to form 8-demethyl-8-hydroxy-5-deazariboflavins.
Chemical properties
Colorless liquid. Slightly soluble in water, miscible with alcohol and oils.
Very diffusive, ethereal, pungent or nauseating odor, pleasant in dilution, slightly wineyfruity, Brandy-like.
Sweet taste in concentrations below 100 ppm.
Barely perceptible at 10 ppm.
The Uses of Trimethoxymethane
Trimethyl Orthoformate is the most simple orthoester. Used in organic synthesis as a reagent for introducing a protecting group for aldehydes and in the creation of methoxymethylene groups and heterocyclic ring systems.
The Uses of Trimethoxymethane
Trimethyl orthoformate is used as a protecting group for aldehydes in organic synthesis, as an additive in polyurethane coatings and as a dehydrating agent in the preparation of surface modified colloidal silica nanoparticles. It is also used as a chemical intermediate in the preparation of vitamin B1 and sulfa drugs. It acts as an effective solvent for thallium(III) nitrate-mediated oxidations. Furthermore. It is utilized for the synthesis of chromone from keto-hydroxy naphthol in the presence of trimethylamine.
The Uses of Trimethoxymethane
Trimethyl orthoformate can be used:
- To convert sulfonic acids to methyl esters.
- To convert 2-acylcyclohexanones to the corresponding acetal derivatives.
- To mediate Pinacol reaction of various 1,2-diols with tin(IV) chloride without the formation of water.
- To synthesize 1-substituted-1H-1,2,3,4-tetrazoles via a three-component condensation with amine and sodium azide catalyzed by indium triflate under solvent-free conditions.
- For the N-methylation of amines in the presence of sulfuric acid.
What are the applications of Application
Trimethyl orthoformate is an effective solvent for thallium(III) nitrate-mediated oxidations
What are the applications of Application
Trimethyl orthoformate was used as dehydrating agent in the preparation of surface-modified colloidal silica nanoparticles.
MOM protection of Diols using Trimethyl Orthoformate
N-Formylation of Amino Acid Esters
General Description
Trimethyl orthoformate is an effective solvent for thallium(III) nitrate-mediated oxidations. It undergoes acid catalyzed reaction with 6-(N-D-ribitylanilino) uracils to form 8-demethyl-8-hydroxy-5-deazariboflavins.
Flammability and Explosibility
Highly flammable
Safety Profile
A skin and eye irritant. A very dangerous fire hazard when exposed to heat or flame; can react with oxidizing materials. Hazardous to prepare. To fight fire, use CO2, fog, haze. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS.
Synthesis
Trimethyl orthoformate is prepared on an industrial scale by the methanolysis of hydrogen cyanide:
HCN + 3 HOCH3 → HC(OCH3)3 + NH3
Trimethyl orthoformate can also be prepared from the reaction between chloroform and sodium methoxide, an example of the Williamson ether synthesis.
Precautions
Moisture sensitive. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with acids and strong oxidizing agents.
References
Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
P. G. M. Wuts, in Greene's Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
A Facile Procedure for the Synthesis of N-Formyl Amino Acid Esters
T. Chancellor, C. Morton, Synthesis 1994, 10, 1023.
Properties of Trimethoxymethane
| Melting point: | -53 °C |
| Boiling point: | 101-102 °C(lit.) |
| Density | 0.97 g/mL at 25 °C(lit.) |
| vapor density | 3.67 (vs air) |
| vapor pressure | 23.5 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 60 °F |
| storage temp. | Store below +30°C. |
| solubility | Miscible with ether, alcohol and benzene. |
| form | Liquid |
| color | Clear colorless |
| explosive limit | 1.4-44.6%(V) |
| Water Solubility | 10 g/L (hydrolysis) |
| Sensitive | Moisture Sensitive |
| Merck | 14,6884 |
| BRN | 969215 |
| CAS DataBase Reference | 149-73-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Methane, trimethoxy-(149-73-5) |
| EPA Substance Registry System | Trimethoxymethane (149-73-5) |
Safety information for Trimethoxymethane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Trimethoxymethane
| InChIKey | PYOKUURKVVELLB-UHFFFAOYSA-N |
Trimethoxymethane manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
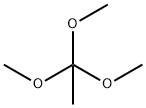
You may like
-
 Trimethyl Orthoformate 98%View Details
Trimethyl Orthoformate 98%View Details -
 Trimethyl orthoformate CAS 149-73-5View Details
Trimethyl orthoformate CAS 149-73-5View Details
149-73-5 -
 Trimethyl Orthoformate CASView Details
Trimethyl Orthoformate CASView Details -
 Trimethyl Orthoformate CASView Details
Trimethyl Orthoformate CASView Details -
 TRI METHYL ORTHO FORMATE, Industrial GradeView Details
TRI METHYL ORTHO FORMATE, Industrial GradeView Details
149-73-5 -
 C4H10O3 Trimethyl OrthoformateView Details
C4H10O3 Trimethyl OrthoformateView Details
149-73-5 -
 Trimethyl Orthoformate CAS No: 149-73-5View Details
Trimethyl Orthoformate CAS No: 149-73-5View Details
149-73-5 -
 Trimethyl Orthoformate TmofView Details
Trimethyl Orthoformate TmofView Details
149-73-5
