Triflumizole
Synonym(s):(E)-4-Chloro-α,α,α-trifluoro-N-(1-imidazol-1-yl-2-propoxyethylidene)-o-toluidine;1-{1-{[4-Chloro-2-(trifluoromethyl)phenyl]imino}-2-propoxyethyl}-1H-imidazole
- CAS NO.:99387-89-0
- Empirical Formula: C15H15ClF3N3O
- Molecular Weight: 345.75
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
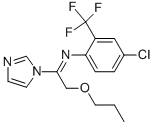
What is Triflumizole?
The Uses of Triflumizole
Triflumizole is used for the control of Gymnospurangum and Venturia spp. in pome fruit, against powdery Erysiphaceae in fruit and vegetables, and against Fusarium, Fulvia and Monilia spp. It is also used as a seed treatment against Helminthospurium, Tilletia and Ustilago spp. in cereals.
Definition
ChEBI: Triflumizole is a carboxamidine resulting from the formal condensation of the amino group of 4-chloro-2-(trifluoromethyl)aniline with the oxygen of the acetyl group of N-(propoxyacetyl)imidazole. A sterol demethylation inhibitor, it is used as a fungicide for the control of powdery mildew, scab and other diseases on a variety of crops. It has a role as an EC 1.14.13.70 (sterol 14alpha-demethylase) inhibitor and an antifungal agrochemical. It is a member of monochlorobenzenes, a member of imidazoles, a member of (trifluoromethyl)benzenes, a carboxamidine, an ether, a conazole fungicide and an imidazole fungicide.
Metabolic pathway
Triflumizole is unstable in aqueous solution when exposed to light and it is readily metabolised by plants and animals. The primary route of metabolism is by cleavage of the imidazole ring to give several metabolites which retain the 4-chloro-2-trifluoromethylaniline moiety. The major metabolite in crops, 2, is formed by loss of the imidazole ring.
Degradation
Aqueous solutions of triflumizole are degraded by sunlight with a DT50 of
29 hours. The sole hydrolysis product of triflumizole in the dark is 2,
which is formed by loss of the imidazole ring.
The major product of photolysis of triflumizole is 4, which is probably
formed in the presence of oxygen via 3. This compound was soluble in the
solid state but decomposed in solution, even if stored in a refrigerator
(Hashimoto et al., 1990).
Properties of Triflumizole
| vapor pressure | 1.86 x 10-4 Pa (25 °C ) |
| pka | 3.7 (base) |
| Water Solubility | 12.5 x 103 mg l-1(20 °C ) |
| EPA Substance Registry System | 1H-Imidazole, 1-[(1E)-1-[[4-chloro-2-(trifluoromethyl) phenyl]imino]-2-propoxyethyl]- (99387-89-0) |
Safety information for Triflumizole
Computed Descriptors for Triflumizole
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1