TRIETHYLENEMELAMINE
- CAS NO.:51-18-3
- Empirical Formula: C9H12N6
- Molecular Weight: 204.23
- MDL number: MFCD00081202
- EINECS: 200-083-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:39
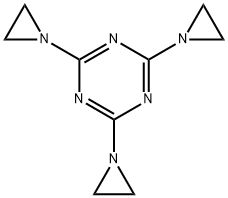
What is TRIETHYLENEMELAMINE?
Originator
Triethylene,Lederle,US,1954
The Uses of TRIETHYLENEMELAMINE
Triethylenemelamine (TEM) is a potent mutagen. It has little, if any, industrial application. TEM is mainly used in medicine as an antineoplastic agent and as a “positive control” in many in vitro and in vivo mutagenicity assays.
The Uses of TRIETHYLENEMELAMINE
manufacture of resinous products, textile finishing agents. Insect sterilant. Research tool used as positive control for mutagenicity assays.
The Uses of TRIETHYLENEMELAMINE
Tretamine is an aziridine chemosterilant that functions as a fumigant.
Definition
ChEBI: Tretamine is a member of 1,3,5-triazines. It has a role as an insect sterilant and an alkylating agent.
Manufacturing Process
Cyanuric chloride (which may or may not contain the usual commercial impurities) is dispersed into ice water by stirring in a ratio of 18.8 g of cyanuric chloride to a mixture of 100 g of ice and 100 g of water. The slurry may conveniently be prepared directly in a 3-necked flask equipped with an agitator, dropping funnel, and thermometer. The temperature of the flask and contents is maintained within the range of 2°C to 5°C, with an ice-salt mixture. A solution of ethylenimine in an aqueous solution of potassium carbonate prepared in the proportions of 14 g ethylenimine, 44.5 g potassium carbonate, and 150 g of water, is added dropwise to the cyanuric chloride slurry. The reaction solution is then clarified with a little activated charcoal, filtered, and extracted with chloroform. Despite the fact that triethylenemelamine is more soluble in water than in chloroform, in a two_x0002_phase system (water-chloroform) nearly 75% of the triethylenemelamine is distributed in the chloroform, and hence a few extractions with that solvent suffice to separate the material from the original reaction medium. Five extractions with 50 ml portions of chloroform gave 19 g of product, and an additional 3 extractions with 25 ml portions gave 0.5 g, a total yield of 95.7%. The product obtained by evaporating such an extract is a white microcrystalline powder.
Therapeutic Function
Antineoplastic
Synthesis Reference(s)
Journal of the American Chemical Society, 77, p. 5915, 1955 DOI: 10.1021/ja01627a040
General Description
Odorless white crystalline powder. Melting point 160°C, then rapidly polymerizes to a white solid. Almost immediate degradation at pH 3.0; rapid degradation at pH 5.0; and very little degradation at pH 7.5.
Air & Water Reactions
Soluble in water [Hawley]. Polymerizes readily with heat or moisture.
Reactivity Profile
TRIETHYLENEMELAMINE polymerizes readily with heat or moisture. Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides.
Fire Hazard
Flash point data for TRIETHYLENEMELAMINE are not available; however, TRIETHYLENEMELAMINE is probably combustible.
Safety Profile
Poison by ingestion, intraperitoneal, intramuscular, intravenous, and subcutaneous routes. Experimental teratogenic and reproductive effects. Questionable carcinogen with experimental neoplastigenic and tumorigenic data. Human mutation data reported. Can cause gastrointestinal tract disturbances and bone marrow depression. When heated to decomposition it emits highly toxic fumes of NOx. Used as an antineoplastic agent and as an insect sterilant.
Carcinogenicity
In several older, limited studies, triethylenemelamine reportedly produced tumors in mice by the dermal and intraperitoneal routes and in rats by subcutaneous and/or intramuscular administration. These data were reviewed and evaluated by the IARC and judged as “limited evidence of carcinogenicity in animals.” As a result of this evaluation of the animal data and a lack of evidence of carcinogenicity in humans, the IARC placed TEM in its Group 3 category.
Properties of TRIETHYLENEMELAMINE
| Melting point: | 160 °C |
| Boiling point: | 332.73°C (rough estimate) |
| Density | 1.2815 (rough estimate) |
| refractive index | 1.7900 (estimate) |
| storage temp. | Refrigerator (+4°C) + Poison room |
| pka | 3.13±0.10(Predicted) |
| Stability: | Unstable - polymerizes at room temperature. Polymerizes more readily if heated and in the presence of moisture. |
| IARC | 3 (Vol. 9, Sup 7) 1987 |
| EPA Substance Registry System | Triethylenemelamine (51-18-3) |
Safety information for TRIETHYLENEMELAMINE
Computed Descriptors for TRIETHYLENEMELAMINE
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4