Altretamine
Synonym(s):2,4,6-Tris(dimethylamino)-1,3,5-triazine;Altretamine;Hexamethylmelamine;N,N,N′,N′,N′′,N′′-Hexamethyl-1,3,5-triazine-2,4,6-triamine
- CAS NO.:645-05-6
- Empirical Formula: C9H18N6
- Molecular Weight: 210.28
- MDL number: MFCD00549245
- EINECS: 211-428-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
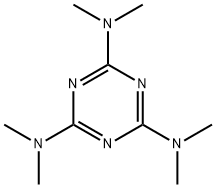
What is Altretamine?
Chemical properties
White Solid
Originator
Hexastat,Roger Bellon,France,1979
The Uses of Altretamine
An antitumor agent which also acts as a chemosterilant for male houseflies and other insects. Antineoplastic.
The Uses of Altretamine
antimalarial
The Uses of Altretamine
As experimental insect chemosterilant.
What are the applications of Application
Altretamine is an antineoplastic
Indications
For use as a single agent in the palliative treatment of patients with persistent or recurrent ovarian cancer following first-line therapy with a cisplatin and/or alkylating agent-based combination.
Background
An alkylating agent proposed as an antineoplastic. It also acts as a chemosterilant for male houseflies and other insects.
Definition
A hexamethyl-2,4,6- triamine derivative of 1,3,5-triazine.
Indications
Although both DNA and RNA synthesis are inhibited in cells exposed to hexamethylmelamine (Hexalen), the molecular mechanisms of these effects are not known.
Manufacturing Process
50 g of hexamethylolmelamine-hexamethyl ether in 950 cc methanol are hydrogenated, at 90°C to 100°C, in the presence of 2 g Raney nickel with 100 atmospheres excess pressure of hydrogen in a steel autoclave holding 2 L until the absorption of hydrogen is terminated. After the catalyst has been filtered off with suction, the methanol is distilled off. As a result, 23.1 g (86% of the theoretical) of crude hexamethylmelamine are formed having a melting point of 158°C to 162°C. After recrystallization from methanol, the pure product is obtained having a melting point of 168°C.
brand name
Hexalen (Millot Laboratories, France).
Therapeutic Function
Antitumor
General Description
Colorless crystalline solid. Insoluble in water.
General Description
Altretamine is available in 50-mg capsules for oraladministration as a second-line treatment for ovarian cancer.The mechanism of action has not been firmly established, althoughthe spectrum of activity is similar to that for otheralkylating agents; however, cross-resistance is not seen.Cytotoxicity has been correlated with metabolism to give thecarbinolamines, which may form imines capable of crosslinking,or decompose to give formaldehyde, which may reactwith nucleophiles on DNA or proteins. The agent is well absorbedupon oral administration, well distributed, and highly(90%) plasma protein bound. The agent is extensively metabolizedin the liver by CYP to give demethylated metabolites via the previously mentioned carbinolamines. Elimination occursprimarily in the urine as mostly demethylated metaboliteswith a terminal elimination half-life of 4 to 10 hours.Myleosuppression and nausea/vomiting are dose-limitingtoxicities. Other adverse effects include lethargy, agitation,hallucinations, skin rash, and elevations in transaminase levels,flulike symptoms, abdominal cramps, and diarrhea.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Altretamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.
Hazard
Toxic.
Mechanism of action
Hexamethylmelamine is readily absorbed after oral administration, with peak plasma levels achieved after 1 hour.The drug is readily metabolized to form a number of demethylated metabolites. Urinary elimination is the primary route of drug excretion.
Pharmacokinetics
Altretamine is a novel antineoplastic agent. The precise mechanism by which altretamine exerts its cytotoxic effect is unknown, although a number of theoretical possibilities have been studied. Structurally, altretamine resembles the alkylating agent triethylenemelamine, yet in vitro tests for alkylating activity of altretamine and its metabolitics have been negative. Altretamine has been demonstrated to be efficacious for certain ovarian tumors resistant to classical alkylating agents. Metabolism of altretamine is a requirement of cytotoxicity. Synthetic monohydroxymethylmelamines, and products of altretamine metabolism, in vitro and in vivo, can form covalent adducts with tissue macromolecules including DNA, but the relevance of these reactions to antitumor activity is unknown.
Clinical Use
Hexamethylmelamine is useful for the treatment of ovarian adenocarcinoma and is frequently combined with cyclophosphamide, cisplatin, and doxorubicin in the treatment of this tumor. It also has some activity against small cell lung cancer.
Side Effects
Nausea and vomiting are the major toxicities associated with hexamethylmelamine administration. Myelosuppression and a peripheral neuropathy also may occur.
Metabolism
Not Available
Metabolism
This unique structure is believed to damage tumor cells through the production of the weakly alkylating species formaldehyde, a product of CYP450-mediated N-demethylation. Administered orally, altretamine is extensively metabolized on first pass, producing primarily mono- and didemethylated metabolites. Additional demethylation reactions occur in tumor cells, releasing formaldehyde in situ before the drug is excreted in the urine.
Properties of Altretamine
| Melting point: | 171-175 °C (lit.) |
| Boiling point: | 339.81°C (rough estimate) |
| Density | 1.0441 (rough estimate) |
| refractive index | 1.6100 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Chloroform (Sparingly), Methanol (Slightly, Heated) |
| form | solid |
| pka | pKa 10.3 (Uncertain) |
| color | White to Off-White |
| Water Solubility | Insoluble |
| Merck | 13,318 |
| CAS DataBase Reference | 645-05-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Altretamine(645-05-6) |
| EPA Substance Registry System | Altretamine (645-05-6) |
Safety information for Altretamine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Altretamine
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Altretamine CAS 645-05-6View Details
Altretamine CAS 645-05-6View Details
645-05-6 -
 Altretamine >98% (HPLC) CAS 645-05-6View Details
Altretamine >98% (HPLC) CAS 645-05-6View Details
645-05-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1